Abstract
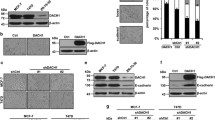
Snail is a denoted transcriptional repressor that plays key roles in epithelial-mesenchymal transition (EMT) and metastasis. Lately, a plethora of genes can be induced by stable expression of Snail in multiple cell lines. However, the biological roles of these upregulated genes are largely elusive. Here, we report identification of a gene encoding the key GlcNAc sulfation enzyme CHST2 is induced by Snail in multiple breast cancer cells. Biologically, CHST2 depletion results in inhibition of breast cancer cell migration and metastasis, while overexpression of CHST2 promotes cell migration and lung metastasis in nude mice. In addition, the expression level of MECA79 antigen is elevated and blocking the cell surface MECA79 antigen with specific antibodies can override cell migration mediated by CHST2 upregulation. Moreover, the sulfation inhibitor sodium chlorate effectively inhibits the cell migration induced by CHST2. Collectively, these data provide novel insights into the biology of Snail/CHST2/MECA79 axis in breast cancer progression and metastasis as well as potential therapeutic strategy for the diagnosis and treatment of breast cancer metastasis.
Similar content being viewed by others
Introduction
Breast cancer has become a primary cause of cancer mortality among women worldwide, and breast cancer metastasis accounts for the majority of cancer-related recurrences and deaths. During metastasis, cancer cells need to complete multistep cell-biological processes, which include matrix proteolysis and invasion into the tumor-associated stroma. Then, tumor cells enter into the lumina of lymphatic or blood vessels and survive in circulation. The final step involves in cancer cells crossing from the lumina vessel into the tissue parenchyma, forming micrometastases in distant organs, and finally proliferating within the microenvironment, leading to the formation of macroscopic metastases [1, 2]. Single-cell RNA sequencing has shown that breast cancer-derived liver and brain metastases exhibit a more complicated intratumoral heterogeneity and immunosuppressive ecosystem, which contribute to immune evasion and tumor malignancy [4, 5]. Snail, in particular, is a well-studied transcription factor and a key regulator of EMT and cancer metastasis involved in multiple mechanisms [6]. Previous studies indicate that Snail promotes tumor progression by conferring tumor cells with stemness traits and promoting tumor cells’ chemoresistance, recurrences, metastasis and cancer-associated fibroblast activation [7,8,9,39]. In this study, we report that the expression of CHST2 contributes to breast cancer metastasis. We discovered breast cancer cell migration and metastasis reduced after depleted CHST2, while cell proliferation is not affected. Conversely, overexpression of CHST2 increased breast cancer cell migration and metastasis in vitro and in vivo. Moreover, cell migration assays indicated that CHST2 is required in Snail-mediated cell metastasis. It is known that in many cellular types overexpression of Snail induces EMT and cell migration, but how much role of EMT contributes to cell migration is unknown. Besides, other mechanisms exist for Snail-mediated cell migration, including formation of lamellipodia upon activation Rac1 and the promotion of neurite outgrowth in prostate cancer[13, 40]. In this study, we discovered CHST2 acts as a novel downstream target to promote Snail-mediated cell migration by increasing cell surface MECA79 antigen synthesis. In addition, sodium chlorate treatment or catalytically inactive mutant can efficiently abolish CHST2 or Snail-mediated cancer cell migration. Those results suggest that CHST2 acts as a critical role in breast cancer metastasis and its function in mediating sialyl LewisX sulfation is responsible for breast cancer metastasis.
The biosynthesis of 6-sulfo sialyl LewisX is a multienzyme catalytic process of glycosylation and sulfation, the mechanisms of glycosylation and post-translational modifications of carbohydrate structures are extremely intricate. Sulfation at the non-reducing C-6 of GlcNAc is essential for L-selectin binding and MECA79 epitope synthesis [41]. Here, we focused on the ubiquitously expressed enzyme CHST2 which catalyzed the transfer of sulfate groups to GlcNAc structures. Our experiments clearly show that CHST2 is responsible for MECA79 antigen synthesis in breast cancer cells. Furthermore, Snail-CHST2 axis mediated sulfation of sialyl LewisX is probably the downstream target to facilitate breast cancer metastasis. The results account for clinical studies which showed ectopic expression of MECA79 antigen on tumor specimens are linked with poor prognosis and metastasis [36, 42, 43]. The carbohydrate antigen sialyl LewisX is a terminal carbohydrate structure which is associated with tumor malignancy. Sialyl LewisX can serve as a tumor marker and alter cancer cell phenotypes by increasing cancer cell motility and proliferation through E-selectin–mediated cancer cell adhesion to vascular endothelial cells or E-selectin-independent pattern [44, 45]. 6-sulfo sialyl LewisX is the sulfation form of sialyl LewisX, in which the GlcNAc is sulfated by specific sulfotransferases. The sulfation modification alters the binding and expression properties of sLeX, making it a ligand for L-selectin, a cell surface receptor expressed on leukocytes [24, 46]. Overall, both sialyl LewisX and 6-sulfo sialyl LewisX are carbohydrate antigens that are associated with tumor malignancy and play important roles in cancer progression and metastasis [47]. However, 6-sulfo sialyl is less well understood in terms of its role in cancer biology. In our study, we tried to elucidate the biological role of 6-sulfation sialyl LewisX in breast cancer and discovered cell surface 6-sulfo sialyl LewisX synthesis mediated by CHST2 is associated with breast cancer metastasis and may be a potential novel breast cancer metastatic marker.
Current findings demonstrate Snail induces CHST2 transcription, which results in cell surface 6-sulfo sialyl LewisX synthesis increase and facilitates migration and metastasis of breast cancer cells. Nevertheless, further studies must be done in the future to elucidate the detailed mechanisms of ectopic MECA79 antigen expression in breast cancer. Previous studies have reported that glycoproteins carrying 6-sulfo sLeX include GlyCAM-1, CD34, MAdCAM-1, PODXL and EMCN, a class of proteins that have mucin-like domains, which act as scaffoldings for O-linked oligosaccharides [48, 49]. Therefore, further investigations will be carried out to identify the glycoproteins and to elaborate on the mechanisms of MECA79 synthesis in breast cancer.
Indeed, studies have demonstrated that 6-sulfo sLeX synthesis in L-selectin ligands requires two 6-sulfotransferases, N-acetylglucosamine-6-O-sulfotransferase-2 (GlcNAc6ST-2, CHST4) which specifically restricted expressed in HEV and ubiquitously expressed CHST2 [22]. In the present study, we have revealed that the expression of CHST2 is induced by Snail in breast cancer cells, while CHST4 expression is not affected by Snail. It has been reported that CHST2 and CHST4 mediated sulfation of sLeX are tissue- and substrate-specific. Specifically, Kenji Uchimura et al. reported CHST2 is the major sulfotransferase enzyme in Peyer’s patches, while in peripheral and mesenteric lymph nodes, CHST4 plays a key role in the synthesis of 6-sulfo sLeX. Those results suggest that CHST2 and CHST4 play complementary roles in 6-sulfo sLeX synthesis in lymph nodes [38]. Here, we reported that Snail-induced expression of CHST2 in breast cancer cells is responsible for 6-sulfo sLeX synthesis, which mediates breast cancer metastasis.
In conclusion, our observations shed light on the molecular mechanisms underlying Snail transactivating CHST2 transcription and expression and elaborate the role of Snail-CHST2 axis in mediating sulfation of sialyl LewisX on the cell surface during breast cancer metastasis. These results provide new insights into Snail function in cancer metastasis. Furthermore, this novel sulfation pathway regulated by Snail may be a promising therapeutic strategy by specifically targeting the MECA79 antigen. In addition, the ectopic expression of the MECA79 antigen will contribute to the diagnosis and treatment of breast cancer.
Materials and methods
Cells and cell culture
The human breast cancer cell lines MDA-MB-231, MCF-7 and non-tumorigenic mammary cells MCF-10A were originally obtained from the American Type Culture Collection (ATCC). Human embryonic kidney cell line 293T, MDA-MB-231 and MCF-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine and penicillin (50 U/ml)/streptomycin (50 μg/ml). MCF-10A cells were maintained in DMEM/F12 supplemented with 5% horse serum, insulin (10 μg/ml), hydrocortisone (0.5 μg/ml), EGF (20 ng/ml), cholera toxin (100 ng/ml) and penicillin/streptomycin. The cell lines were authenticated by DNA fingerprinting in the Shanghai Jiao Tong University Analysis Core and were cultured in a 37 °C water-saturated 5% CO2 humidified chamber.
Plasmids
The human CHST2 cDNA was subcloned into pCDH-CMV-MCS-EF1-Puro-vector by PCR technique with EcoR I and Not I restriction enzyme sites. The pCDH-Flag-CHST2-N475A variant were cloned from pCDH-Flag-CHST2 by point mutation of the amino acid residue 475 N into A. The CHST2 gene promoter spanning from −638 to +426 was generated from genomic DNA of the human embryonic kidney cell line 293T by PCR and subcloned into pGL3.0-Luc basic vector, generating the pGL3-CHST2-Luc reporter constructs. The pLU-Flag-Snail plasmid has been previously described [34]. The short hairpin RNA sequences of pLKO.1- shCHST2 were obtained from GE-Life science and are listed in Table 1.
RNA extraction and RT-PCR analysis
Total RNA was extracted following standard protocols with TRIzol reagent (Ambion, Carlsbad, CA, USA). Complementary DNAs were synthesized with 3 μg of total RNA using iScript cDNA Synthesis Kit (Fermentas, San Jose, CA, USA). The detailed procedures of RNA extraction and qRT-PCR were previously described [50]. The qRT-PCR assays were performed with SYBR green reagent (ABI 7500 Fast).
Western blots and antibodies
Protein samples were prepared from various cells at 60~80% confluency. The cells were washed in ice-cold PBS, and then lysed in ice-cold lysis buffer containing protease inhibitor phenylmethylsulfonyl fluoride (PMSF) and cocktail, 25 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% NP40, 1 mM EDTA. Cell lysates were then clarified by centrifugation, protein concentrations were determined using the bicinchoninic acid (BCA) Protein Assay Kit (Thermo Scientific, USA). The antibodies were used in western blots as follows: CHST2 antibody (26027-1-AP, Proteintech, Chicago, IL, USA), Snail antibody (3879S, Cell Signaling Technology, Massachusetts, USA), anti-Flag (F3165, F7425, Sigma, St. Louis, MO, USA), Alpha Tubulin antibody (66031-1-Ig, Proteintech, Chicago, IL, USA), GAPDH antibody (60004-1-Ig, Proteintech, Chicago, IL, USA), Normal rabbit IgG (sc-2027, Santa Cruz).
Transfection, luciferase reporter assays and ChIP
For transfection, 293T cells were seeded at a density of 5 × 104 cells per well in 24-well plates. The β-galactosidase plasmids (10 ng) and pGL3-CHST2-Luc reporter (100 ng), along with Snail-encoding plasmids, were transiently transfected into the cells with Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA). The following procedures were performed as described [34]. ChIP assay was performed according to published protocols [34] in MDA-MB-231 cells and MCF-10A stably expressing Snail cells with slight modifications. To prepare cells for ChIP, MDA-MB-231 cells and MCF-10A-Snail cells were grown in 100-mm plates to 70–80% confluence and fixed by the addition of 287 μl of 37% formaldehyde directly into 10 ml of growth medium to a final concentration of 1% for 15 min at room temperature. The cross-linking reaction was stopped by the addition of 625 μl of 2 M glycine in phosphate-buffered saline buffer at room temperature for 5 min. 1 × 107 cells were harvested, the chromatin was sonicated into fragments ranging from 500 to 800 bps in size, the sonication fragments were resolved on 2% agarose gels and visualized with ethidium bromide. The immunoprecipitated DNAs were amplified by real-time PCR with primer sets listed in Table 1.
Cell migration and invasion assays
Migration of breast cancer cells was determined using 24-well Boyden chambers (Corning) with 8 μm-inserts. The detailed procedures of migration assay were previously described [17, 51]. Exponentially growing MDA-MB-231, MCF-10A or MCF-7 cells were digested by trypsin and collected by centrifugation. The cells were resuspended in serum-free DMEM or DMEM/F12 blank medium and counted. 2.5 × 104 of MDA-MB-231 or 5 × 104 of MCF-10A or 4 × 104 of MCF-7 cells in 100 μl serum-free DMEM or DMEM/F12 blank medium were seeded on 8 μm-inserts, then 600 μl DMEM or DMEM/F12 complete growth medium were added to the bottom chamber as attractants. After incubation for 20–24 h, the cells were fixed with 4% paraformaldehyde and stained with crystal violet, non-migrated cells on the top of the chamber were removed gently with cotton swabs, then migrated cells were counted as per field of view under phase-contrast microscopy. For invasion assay, the matrigel matrix (Corning, #354234) was diluted to 250 μg/ml on ice, and 100 μl diluted matrigel was added into 8 μm-inserts. Then the transwell filter was placed in the 37 °C incubator for 2 h until the matrigel solidified. The following procedures are similar to migration assays. The number of migrated cells was calculated by the relative cell migration area through Image-J software.
Wound-healing assays
MCF-10A cells were stably expressed with Flag-CHST2 and Flag-CHST2-N475A individually, then, subconfluent growing cells were digested by trypsin and counted using hemocytometer, 5 × 105 cells were seeded onto a 6-well tissue culture plate. After incubating the seeded cells for 6 h at incubator with complete DMEM/F12 medium, the confluent monolayer cells were scratched in a straight line to create a “scratch” with a 10 μl pipette tip, removing the cell debris and smooth the edge of the scratch by washing the cells twice with 2 ml of the PBS, and then added 2 ml DMEM/F12 medium supplemented with 1% horse serum, insulin (2 μg/ml), hydrocortisone (0.1 μg/ml), EGF (4 ng/ml) and cholera toxin (20 ng/ml). And afterwards, creating markings on the outer bottom of the dish with an ultrafine tip marker. Then 0 h images were captured using a phase-contrast microscope. Place the plates in incubator at 37 °C under 5% CO2 for 24 h, the second images were captured of the same wounds at 24 h. The scratch areas were measured and presented as the percentage of scratch areas at 0 h (mean ± SD). The percent of migration was calculated as the migration value of the scratch areas at 24 h divided by the scratch areas at 0 h. Each sample was performed at least three times [52].
Cell proliferation and cell viability
Cell proliferation rate and cell viability were measured by cell counting kit-8 (C0038, Beyotime). For cell proliferation analysis, seed cells in a 96-well plate at a density of 1 × 103 cells per well in 100 μl of culture medium and incubate the plate in the incubator for ~12 h, then add 10 μl of CCK8 solution to each well and incubate another 1 hour, measure the absorbance at 450 nm every other day to determine the cell proliferation rate. For cell viability assay, 5 × 103 cells were seeded onto a 96-well plate, then incubate the plate for 4~6 h in a humidified incubator at 37 °C, 5% CO2, and after cells were treated with 0, 5 and 10 mM sodium chlorate for 24 h, add 10 μl of CCK8 solution to each well with another 1-hour incubation in the incubator, the absorbance of 450 nm was tested to determine cell viabilities.
Mouse experiments
Female BALB/c nude mice were purchased from SLAC laboratory Co. Ltd (Shanghai, China) and divided into two groups randomly. To establish in vivo lung metastatic mouse models of MDA-MB-231 cells, Exponentially growing MDA-MB-231 cells (labeled with luciferase reporter) were digested and resuspended in PBS buffer, then 1 × 106 of MDA-MB-231-sh-CHST2/sh-Vector or 5 × 105 of MDA-MB-231-Flag-CHST2/Vector cells in 100 μl of PBS suspensions were injected into the tail vein of 6-week-old mice. Lung micro-metastatic foci were analyzed by using the Xenogen IVIS Imaging System (PerkinElmer). Animal studies were conducted following the Institutional Animal Care and Use Committee of Shanghai in accordance with the National Research Council Guide for Care and Use of Laboratory Animals (SCXK, Shanghai 2007-0005). All mice were fed under specific pathogen-free (SPF) conditions. To ameliorate any suffering, mice were euthanized by CO2 inhalation.
Hematoxylin-eosin staining and immunohistochemistry assays
After lung metastatic mice models were analyzed at the endpoint, the mice were euthanized and lungs with metastatic foci were dissected and fixed with 5 ml of 4% paraformaldehyde. Tissues were then embedded in paraffin blocks, and sections were cut (4 μm) for H&E staining and immunohistochemistry. For immunohistochemical staining, lung sections were baked at 65 °C overnight, then de-paraffinized by three 10-min extractions in xylene, followed by a series of steps de-paraffinized using 100%, 100%, 90%, 90% and 75% ethanol for 5 min each. Then tissue slides are soaked in gently running tap water for 30 min. Afterwards, sections were transferred to boiling sodium citrate buffer (pH 6.0) for antigen retrieval for 30 min and then cooled to room temperature. Then, sections were pre-treated with 3% hydrogen peroxide for 15 min before blocking. Blocking was performed with 5% bovine serum albumin in PBS for 30 min at room temperature followed by primary antibody incubation overnight at 4 °C. Immunoreaction was detected using SABC-AP assay (Boster, SA1050) according to the manufacturer’s instructions. The sections were visualized under a microscope. The following primary antibodies were used: rat monoclonal MECA‐79 antibody (sc‐19602, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA).
Flow‑cytometric analysis
MDA-MB-231, MCF-7 and MCF-10A cells were harvested at a subconfluent stage, then the cells were collected by centrifugation for 5 min at 800 × g, at 4 °C, washed twice and resuspended in PBS containing 1% FBS at 1 × 106 cells/ml density. Subsequently, the cell suspensions were incubated with each mAb (MECA79 (rat mAb, Santa Cruz Biotechnology) or normal rat IgM (sc-3885, Santa Cruz Biotechnology) for 30 min on ice, followed by staining with APC-conjugated goat anti-rat IgM (A10540, Invitrogen, Carlsbad, CA, USA) or FITC-conjugated sheep anti-rat (PA1-28638, Invitrogen, Carlsbad, CA, USA) IgG for 30 min on ice, and then analyzed on a FACScan (CytoFLEX LX, Beckman Coulter, USA). Experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA) and Graph Pad Prism 7.0 (Graph Pad Software, Inc., La Jolla, CA, USA) software. The independent student’s t tests were used for comparison between two groups. Data are presented as the mean ± SD. The correlation between the expression of Snail and CHST2 in breast tumor samples was evaluated by the Pearson rank correlation coefficient test. P < 0.05 was considered to indicate a statistically significant difference.
Data availability
The data, protocols and sequences that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Zou Y, Ye F, Kong Y, Hu X, Deng X, **e J, et al. The single-cell landscape of intratumoral heterogeneity and the immunosuppressive microenvironment in liver and brain metastases of breast cancer. Adv Sci. 2023;10:e2203699.
Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412.
Liu P, Wang Z, Ou X, Wu P, Zhang Y, Wu S, et al. The FUS/circEZH2/KLF5/ feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol Cancer. 2022;21:198.
Wu Y, Zhou BP. Snail: More than EMT. Cell Adhes Migr. 2010;4:199–203.
Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–72.
De Craene B, Berx G. Snail in the frame of malignant tumor recurrence. Breast Cancer Res. 2006;8:105.
Hojo N, Huisken AL, Wang H, Chirshev E. Snail knockdown reverses stemness and inhibits tumour growth in ovarian cancer. Sci Rep. 2018;8:8704.
Cai F, **ao H, Sun Y, Wang D, Tang J. Expression of snail and E-cadherin in drug-resistant MCF-7/ADM breast cancer cell strains. J Coll Physicians Surg Pak. 2019;29:240–4.
Alba-Castellón L, Olivera-Salguero R, Mestre-Farrera A, Peña R, Herrera M, Bonilla F, et al. Snail1-dependent activation of cancer-associated fibroblast controls epithelial tumor cell invasion and metastasis. Cancer Res. 2016;76:6205–17.
Li CF, Chen JY, Ho YH, Hsu WH, Wu LC, Lan HY, et al. Snail-induced claudin-11 prompts collective migration for tumour progression. Nat Cell Biol. 2019;21:251–62.
Edwards G, Campbell T, Henderson V, Danaher A, Wu D, Srinivasan R, et al. SNAIL transctiption factor in prostate cancer cells promotes neurite outgrowth. Biochimie. 2021;180:1–9.
Jordà M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–85.
Hsu DS, Lan HY, Huang CH, Tai SK, Chang SY, Tsai TL, et al. Regulation of excision repair cross-complementation group 1 by Snail contributes to cisplatin resistance in head and neck cancer. Clin Cancer Res. 2010;16:4561–71.
Tao G, Levay AK, Gridley T, Lincoln J. Mmp15 is a direct target of Snai1 during endothelial to mesenchymal transformation and endocardial cushion development. Dev Biol. 2011;359:209–21.
Zhang Y, Zou X, Qian W, Weng X, Zhang L, Zhang L, et al. Enhanced PAPSS2/VCAN sulfation axis is essential for Snail-mediated breast cancer cell migration and metastasis. Cell Death Differ. 2019;26:565–79.
Stanisavljevic J, Porta-de-la-Riva M, Batlle R, de Herreros AG, Baulida J. The p65 subunit of NF-κB and PARP1 assist Snail1 in activating fibronectin transcription. J Cell Sci. 2011;124:4161–71.
Uchimura K, El-Fasakhany FM, Hori M, Hemmerich S, Blink SE, Kansas GS, et al. Specificities of N-acetylglucosamine-6-O-sulfotransferases in relation to L-selectin ligand synthesis and tumor-associated enzyme expression. J Biol Chem. 2002;277:3979–84.
Fujiwara M, Kobayashi M, Hoshino H, Uchimura K, Nakada T, Masumoto J, et al. Expression of long-form N-acetylglucosamine-6-O-sulfotransferase 1 in human high endothelial venules. J Histochem Cytochem. 2012;60:397–407.
Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, et al. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci USA. 2006;103:13333–8.
Li X, Tu L, Murphy PG, Kadono T, Steeber DA, Tedder TF. CHST1 and CHST2 sulfotransferase expression by vascular endothelial cells regulates shear-resistant leukocyte rolling via L-selectin. J Leukoc Biol. 2001;69:565–74.
Grunwell JR, Bertozzi CR. Carbohydrate sulfotransferases of the GalNAc/Gal/GlcNAc6ST family. Biochemistry. 2002;41:13117–26.
Uchimura K, Kadomatsu K, El-Fasakhany FM, Singer MS, Izawa M, Kannagi R, et al. N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing. J Biol Chem. 2004;279:35001–8.
Chen L, Ichihara-Tanaka K, Muramatsu T. Role of the carboxyl-terminal region in the activity of N-acetylglucosamine 6-o-sulfotransferase-1. J Biochem. 2004;136:659–64.
Uchimura K, Gauguet J-M, Singer MS, Tsay D, Kannagi R, Muramatsu T, et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol. 2005;6:1105.
Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6:1096.
Kawashima H, Fukuda M. Sulfated glycans control lymphocyte homing. Ann N Y Acad Sci. 2012;1253:112–21.
Girard JP, Amalric F. Biosynthesis of sulfated L-selectin ligands in human high endothelial venules (HEV). Adv Exp Med Biol. 1998;435:55–62.
Desko MM, Gross DA, Kohler JJ. Effects of N-glycosylation on the activity and localization of GlcNAc-6-sulfotransferase 1. Glycobiology. 2009;19:1068–77.
Nandini CD, Sugahara K. Role of the sulfation pattern of chondroitin sulfate in its biological activities and in the binding of growth factors. Adv Pharmacol. 2006;53:253–79.
Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–19.
Herranz N, Pasini D, Díaz VM, Francí C, Gutierrez A, Dave N, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–81.
Hou Z, Peng H, Ayyanathan K, Yan KP, Langer EM, Longmore GD, et al. The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol. 2008;28:3198–207.
Lin T, Ponn A, Hu X, Law BK, Lu J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene. 2010;29:4896–904.
Okayama H, Kumamoto K, Saitou K, Hayase S, Kofunato Y, Sato Y, et al. Ectopic expression of MECA-79 as a novel prognostic indicator in gastric cancer. Cancer Sci. 2011;102:1088–94.
Hoshino H, Foyez T, Ohtake-Niimi S, Takeda-Uchimura Y, Michikawa M, Kadomatsu K, et al. KSGal6ST is essential for the 6-sulfation of galactose within keratan sulfate in early postnatal brain. J Histochem Cytochem. 2014;62:145–56.
Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K. N-Acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology. 2006;16:702–10.
Uchimura K, Muramatsu H, Kaname T, Ogawa H, Yamakawa T, Fan QW, et al. Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis X: molecular cloning, chromosomal map**, and expression in various organs and tumor cells. J Biochem. 1998;124:670–8.
Henderson V, Smith B, Burton LJ, Randle D, Morris M, Odero-Marah VA. Snail promotes cell migration through PI3K/AKT-dependent Rac1 activation as well as PI3K/AKT-independent pathways during prostate cancer progression. Cell Adhes Migr. 2015;9:255–64.
Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–56.
Hoshino H, Ohta M, Ito M, Uchimura K, Sakai Y, Uehara T, et al. Apical membrane expression of distinct sulfated glycans represents a novel marker of cholangiolocellular carcinoma. Lab Investig. 2016;96:1246–55.
Taga M, Hoshino H, Low S, Imamura Y, Ito H, Yokoyama O, et al. A potential role for 6-sulfo sialyl Lewis X in metastasis of bladder urothelial carcinoma. Urol Oncol. 2015;33:496.e1-9.
Guerrero PE, Miró L, Wong BS, Massaguer A, Martínez-Bosch N, Llorens R, et al. Knockdown of α2,3-sialyltransferases impairs pancreatic cancer cell migration, invasion and E-selectin-dependent adhesion. Int J Mol Sci. 2020;21:6239.
Dall’Olio F, Pucci M, Malagolini N. The cancer-associated antigens sialyl Lewis(a/x) and Sd(a): two opposite faces of terminal glycosylation. Cancers. 2021;13:5273.
Bowman KG, Cook BN, de Graffenried CL, Bertozzi CR. Biosynthesis of L-selectin ligands: sulfation of sialyl Lewis x-related oligosaccharides by a family of GlcNAc-6-sulfotransferases. Biochemistry. 2001;40:5382–91.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55.
Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet JM, Yu SY, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–18.
Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–69.
Li Q, Peng H, Fan H, Zou X, Liu Q, Zhang Y, et al. The LIM protein Ajuba promotes adipogenesis by enhancing PPARγ and p300/CBP interaction. Cell Death Differ. 2016;23:158–68.
Qian W, Li Q, Wu X, Li W, Li Q, Zhang J, et al. Deubiquitinase USP29 promotes gastric cancer cell migration by cooperating with phosphatase SCP1 to stabilize Snail protein. Oncogene. 2020;39:6802–15.
Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods Mol Biol. 2005;294:23–9.
Acknowledgements
The authors express their gratitude to diverse facilities from Shanghai Jiaotong University School of Medicine: Core Facility of Basic Medical Sciences, the animal housing facility and the cytometry platform, especially to Yong Zhou, **g Zhou, Gui** Li and Naian Shen for their technical support.
Funding
This work was supported by the Shandong Provincial Natural Science Foundation (ZR2021ZD16); the National Natural Science Foundation of China (82273392, 81903003); Shanghai Frontiers Science Center of Cellular Homeostasis and Human Diseases.
Author information
Authors and Affiliations
Contributions
The project was conceived by ZYH, CYD; Most of experiments were designed and performed by DZ and YHZ with assistance from XQZ, MYL, HZ, YND, JMW, CCP; Software and data analysis: DZ and YHZ; Funding acquisition: YHZ; ZYH and DZ wrote the Original Draft; CYD, ZYH and DZ reviewed and edited the manuscript. All authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University School of Medicine.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Anastasis Stephanou
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, D., Zhang, Y., Zou, X. et al. CHST2-mediated sulfation of MECA79 antigens is critical for breast cancer cell migration and metastasis. Cell Death Dis 14, 288 (2023). https://doi.org/10.1038/s41419-023-05797-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-05797-x
- Springer Nature Limited