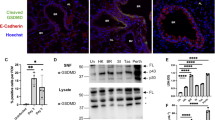
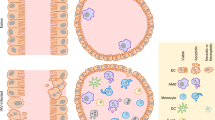
Abstract
Influenza virus is one of the most challenging viruses threating human health. Since infection with influenza virus triggers inflammatory responses and induces cell death, the molecular and cellular mechanisms by which the virus-infected cells undergo apoptotic and necrotic cell death have been widely studied. However, most of the studies have focused on the molecular events occurring in the cytosol and there is limited information on the physiological correlation between virus-induced cell death and the viral pathogenesis in vivo. In this study, we demonstrate that the influenza virus matrix 1 (M1) protein is released from virus-infected cells and triggers apoptotic cell death of lung epithelial and pulmonary immune cells, through the activation of Toll-like receptor 4 (TLR4) signaling. Treatment with M1 protein led to robust cellular inflammatory responses, such as the production of proinflammatory cytokines and cellular reactive oxygen species (ROS), and induction of cell death. When M1 protein was administered in vivo, it induced the activation of inflammatory responses and cell death in the lungs. Furthermore, the administration of M1 aggravated lung pathology and mortality of the virus-infected mice in a TLR4-dependent manner. These results demonstrate that M1 is an important pathogenic factor contributing to influenza virus pathogenicity by enhancing cell death in the lungs, thereby expanding our understanding of the molecular mechanism of influenza virus-induced cell death through the interaction with an innate immune receptor.
Similar content being viewed by others
Introduction
Influenza is an acute respiratory viral disease with approximately 3 to 5 million cases of severe illness and up to 650000 deaths annually, worldwide. Therefore, the underlying mechanisms of the pathogenesis of the influenza virus have attracted extensive attention. Previous reports have demonstrated that influenza virus triggers various inflammatory responses, such as the upregulation of proinflammatory cytokines and chemokines, overproduction of intracellular reactive oxygen species (ROS), and enhanced programmed cell death [1,2,3]. Since these cellular responses can lead to severe lung injury, identification of precise inflammation-associated factors and pathways is important for understanding the pathogenesis of influenza and develo** novel therapeutic strategies.
Acute viral infection is commonly associated with cell death. Since lung epithelial and pulmonary immune cells directly infected with influenza virus undergo apoptotic, necrotic and necroptotic cell death, the underlying molecular mechanisms have been widely studied [4,5,6,7]. Particularly, intracellular interactions between viral and host proteins have attracted the most attention because it has been regarded that viral proteins, especially internal proteins, are expressed and reside in the cytosol. However, it was recently reported that nucleoprotein (NP) can be released from infected cells and increase viral pathogenicity through the activation of Toll-like receptor 4 (TLR4), a membrane receptor, on innate immune cells in the lungs [8]. This suggests a potential role for viral internal proteins in the disease pathogenesis through the interaction with host cells in the extracellular compartment.
Influenza virus matrix 1 (M1) protein is important for viral replication and sha** of virus particles [26,27,28,29]. It is also controversial whether the activation of the NLRP3 inflammasome and production of IL-1β and IL-18 increases or decreases lung pathology [30,31,32]. In this work, we demonstrated that TLR4 recognizes extracellular M1 and aggravates viral pathogenicity in vivo. Interestingly, inactivated H5N1 virus induced acute lung injury in a TLR4-dependent manner, partially supporting our findings [12]. Furthermore, we recently reported the pathogenic role of TLR4 through the interaction with influenza virus NP [8]. Collectively, TLR4 plays a pathogenic role in the pathogenesis of influenza by recognizing a wide range of ligands from oxidized phospholipid to viral proteins.
Excess levels of ROS can lead to mitochondrial damage and consequent apoptotic cell death. In the case of influenza viral infection, oxidative stress by ROS provokes inflammation and acute lung injury [12, 33, 34]. It is well known that oxidized phospholipid can trigger ROS production through the activation of TLR4 [12]. In this study, we additionally showed that M1, the most abundant influenza viral protein, can be another trigger for ROS production in vitro and in vivo. However, it is intriguing that cellular ROS is also required for the optimal induction of the antigen-specific CD8+ T cell response by increasing antigen cross-presentation by dendritic cells [35,36,37]. In our study, we did not investigate the effect of M1 on the fate or behavior of dendritic cells. It is worthwhile to study whether and how M1 differentially modulates the host response to the viral infection depending on physiological conditions such as its micro-concentration and the cell types it encounters.
The activation of TLR4 has different sub-signaling mechanisms depending on the type of ligand [38, 39]. Bacteria-derived LPS activates both MyD88-dependent and TRIF-dependent pathways [38], but monophosphoryl lipid A (MPLA), a low-toxic derivative of lipid A moiety of LPS, predominantly activates the TRIF-dependent pathway [40, 41]. Consequently, TLR4 activation by LPS and MPLA differs in the induction of transcriptome change, chemokine production, inflammation, and memory CD8+ T cell differentiation [42, 43]. Thus, identifying novel ligands of TLR4 and their biological consequence is of continuing interest. Our gene expression profile results showed that M1 and LPS differentially activated downstream target genes, and, interestingly, it did not match those prompted by NP (Figs. 1F and 3F, and Fig. S7A–C). Therefore, further study is required on ligand dependent TLR4 signaling mechanisms in terms of molecular and structural biology.
Previous studies have demonstrated that anti-M1 antibody cannot directly neutralize influenza virus [44]. However, our data showed that anti-M1 antibody alleviates disease severity and improves survival rate against the viral challenge. Anti-M1 plasma or anti-M1 antibody reduced the level of inflammatory cytokines and cell death in infected mice. Antibodies to NP have shown an antiviral effect against influenza virus infection in murine models [45,46,47]. Although it has been previously suggested that NK cells contribute to the antiviral effect via antibody-dependent cellular cytotoxicity, the exact mechanism has not been experimentally proved. In this study, we did not investigate the possibility of involvement of NK cells in the anti-M1 antibody-mediated therapeutic effect. Nonetheless, antibodies targeting M1 could be an auxiliary therapeutic approach for influenza.
In conclusion, we showed that M1 is an important pathogenic factor contributing to influenza viral pathogenicity by increasing ROS-mediated cell death in the lungs. Intriguingly, M1 is released from infected cells to the extracellular compartment and interacts with TLR4, a membrane receptor protein, on the lung epithelial and pulmonary immune cells. This finding not only expands our understanding of the molecular mechanism of influenza virus-induced cell death and viral pathogenesis but could also be useful information for the development of a novel antiviral drug.
Materials and methods
Mice
Seven- to eight-week-old C57BL/6 and BALB/c female mice were purchased from KOATECH (Pyeongtaek, Korea) and maintained in a specific pathogen-free biosafety level-2 facility at the Korea Research Institute of Bioscience and Biotechnology (KRIBB). TLR4 knockout mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). Only 8- to 12-week-old female mice were used in this study. All animal experiments were approved by the Institutional Animal Use and Care Committee of KRIBB (KRIBB-AEC-19173) and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Cell culture
HEK-Blue™ TLR4 (InvivoGen, San Diego, CA, USA) and L929 (ATCC, Manassas, Virginia, USA) cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, Utah, USA) and 1× antibiotics (Gibco™, Massachusetts, USA). MLE-12 cells (ATCC) were maintained in DMEM supplemented with 2% FBS and 1× antibiotics. No mycoplasma contamination was detected. To prepare BMDMs, bone marrow cells were harvested from the femur and tibia of C57BL/6 N and TLR4 knockout mice and differentiated in DMEM containing 25% L929-conditioned medium, 10% FBS, and 1× antibiotics. L929-conditioned medium was obtained by culturing L929 cells in DMEM for 7 days until a confluence of >90% was obtained. Afterwards, the medium underwent centrifugation at 1500 rpm for 10 min and the supernatants were collected and filtered through a 0.45 μm filter system.
Recombinant M1 protein
The DNA fragment encoding M1 protein (GenBank: CY147535.1) was cloned into the pEXPR-IBA 103 vector (IBA Lifesciences, Göttingen, Germany), and the plasmid DNA was transfected into ExpiCHO cells. Five days later, the cells were harvested and lysed by sonication (30% amplitude, 10 s on/10 s off, total 10 min). After filtration with a 0.45 μm filter (Corning), the lysate supernatant was bound to Strep-Tactin XT resin (IBA Lifesciences) using a gravity flow column. After washing, the bound protein was collected by treating the column with an elution buffer (IBA Lifesciences). The endotoxin level in the purified M1 was measured using the endotoxin test kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The protein was stored at –80 °C until further use.
Virus
The influenza A/Puerto Rico/8/1934 (PR8) virus was cultivated in the allantoic cavity of embryonated chicken eggs. Viruses were titrated by calculating the 50% egg infectious dose (EID50) and 50% tissue culture infectious dose (TCID50) and stored at –80 °C until use.
Anti-M1 polyserum and anti-M1 antibody
The M1-coding DNA fragment was cloned into the pGX-10 plasmid [48], which was used as the DNA vaccine vector in this study. Mice (BALB/c) were intramuscularly immunized twice at 3-week intervals with the plasmid DNA (10 μg) using electroporation. Serum was obtained 2 weeks after the final vaccination. Anti-M1 IgG was purified from the serum of mock- and M1-vaccinated mice using an IgG antibody purification kit (Abcam, Cambridge, UK) according to the manufacturer’s protocol. Serum and purified antibodies were stored at –20 °C.
Preparation of lung samples (whole lung cells, BAL fluid, and BAL cells)
Total lung cells and BAL fluid samples were obtained as described previously [8]. Briefly, lung tissue was minced and incubated in 1.5 ml RPMI 1640 media containing collagenase D (150 unit/ml, Gibco), DNase I (50 μg/ml, Merck), 10% FBS, and 1× antibiotics. After enzymatic digestion, cells were isolated from the tissue using a strainer (SPL, Pyeongtaek, Korea). For BAL fluid preparation, a catheter was inserted in the trachea of anesthetized mice and 1 ml of cold PBS containing protease inhibitors (Merck) was instilled into the lungs. After gentle aspiration, BAL fluid and cells were separated by centrifugation (4000 rpm, 5 min, 4 °C).
Experimental schedule
Mice (C57BL/6) were treated intranasally with recombinant M1 protein (rM1), after which, BAL fluid, BAL cells and lung cells were obtained at each indicated time point for enzyme-linked immunosorbent assay (ELISA), western blotting, and flow cytometry analysis. Mice (C57BL/6) infected with PR8 influenza virus (32 PFU) were intranasally treated with PBS or M1 immediately, 3 days pre- or post-infection. Influenza virus-infected mice were intraperitoneally administered with 200 μl of naïve or anti-M1 serum or 15–200 μg of anti-M1 purified IgG antibody daily for 3 days. The change in bodyweight and survival rate of the mice were monitored for 14 days post infection.
Western blot
Cells were lysed in CETi lysis buffer (TransLab, Daejeon, Korea) and the lysate was separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transferring the proteins onto polyvinylidene fluoride membranes (Merck), the membranes were blocked and incubated with primary antibodies overnight at 4 °C. After washing, horseradish peroxidase (HRP)-conjugated secondary antibodies were added. Bound antibodies were visualized using Immobilon Forte Western HRP substrate (Merck) and imaged using EZ-Capture II (ATTO Corporation, Tokyo, Japan). The following antibodies were used: anti-phospho-SAPK/JNK (Cat# 9255), anti-SAPK/JNK (Cat# 9252), anti-phospho-p44/42 MAPK (Cat# 4370), anti-p44/42 (Cat# 4695), anti-phospho-p38 MAPK (Cat# 4511), anti-p38 MAPK (Cat# 54470), anti-phospho-IκB (Cat# 2859), anti-IκB-alpha (Cat# 4812), anti-phospho-NF-κB p65 (Cat# 3033), anti-COX1 (Cat# 9896), anti-COX2 (Cat# 12282), anti-iNOS (Cat# 13120), anti-Bim (Cat# 2933), anti-phospho-Bad (Cat# 5284), anti-Bak (Cat# 12105), anti-caspase-3 (Cat# 9665), anti-caspase-6 (Cat# 9762), anti-caspase-7 (Cat# 8438), anti-caspase-9 (Cat# 9508), HRP-linked anti-mouse IgG (Cat# 7076), HRP-linked anti-rabbit IgG (Cat# 7074),(all from CST, Danvers, Massachusetts, USA), and anti-Strep-tag II (Abcam, Cat# ab76949).
IP
For IP, BMDMs were lysed in CETi lysis buffer for 10 min on ice. Lysates were centrifuged and the supernatants were transferred to fresh tubes containing antibody coated Dynabeads as per the manufacturer’s instructions (Invitrogen), followed by western blot analysis. The following antibodies were used: anti-TLR4 (Santa Cruz Biotechnology, Dallas, Texas, USA; and Thermo Fisher Scientific, Cat# sc-293072) and anti-influenza M1 (Sino biological, Bei**g, China, Cat# 40010-RP01).
ELISA, LDH, and ROS assay
BMDM, MLE-12, and BAL cell culture supernatants were collected after treatment with M1 or LPS for 20 h for ELISA and after 0–48 h for LDH and ROS assays. BAL fluid samples were obtained from the mice, and cytokines were measured using IL-6, CXCL1, CXCL-10, CCL2, CCL5 ELISA kits (all from R&D Systems, Minneapolis, Minnesota, USA). LDH and ROS were detected using the CyQUANT™ LDH cytotoxicity assay (Invitrogen) and in vitro ROS/RNS assay kits (Cell Biolabs Inc. San Diego, CA, US), respectively, according to the manufacturers’ protocols.
RNA sequencing and analysis
(1) RNA isolation
BMDM cells were treated with PBS, M1, NP, or LPS for 6 h, after which total RNA was isolated using Trizol reagent (Invitrogen Corp., Carlsbad, CA, USA). RNA purity and integrity were evaluated using a ND-2000 Spectrophotometer (NanoDrop, Wilmington, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, USA), respectively.
(2) Affymetrix whole-transcript expression array
The Affymetrix Whole-transcript Expression array was performed using the GeneChip Whole Transcript PLUS reagent Kit (Thermo Fisher Scientific) per the manufacturer’s protocol. cDNA was synthesized using the GeneChip WT (Whole Transcript) Amplification kit as described by the manufacturer. The sense cDNA was then fragmented and biotin-labeled with terminal deoxynucleotidyl transferase (TdT) using the GeneChip WT Terminal labeling kit. Approximately 5.5 μg of labeled DNA target was hybridized to the Affymetrix GeneChip Mouse ST 2.0 Array at 45 °C for 16 h. Hybridized arrays were washed and stained on a GeneChip Fluidics Station 450 and scanned on a GCS3000 Scanner (Affymetrix). Signal values were computed using the Affymetrix® GeneChip™ Command Console software.
(3) Data analysis
Expression data were generated by Transcriptome Analysis console 4.0.1. For the normalization, robust multi-average (RMA) algorithm implemented in Transcriptome Analysis Console software was used. Data mining and graphic visualization were performed using ExDEGA (Ebiogen Inc., Korea). To define differentially expressed genes (DEGs), adjusted |log2fold-change (FC)| ≥ 1.5 and P < 0.05 were selected as cut-off values.
TLR reporter cell line assay
The TLR reporter cell lines (Invivogen) were cultured in DMEM containing 10% FBS and 1× antibiotics. When the HEK-Blue™ TLR4 reporter cells reached 80% confluency, they were harvested using a scraper and resuspended in HEK-Blue™ Detection media (InvivoGen). The resuspended cells (5 × 104 cells/well; 180 μl) were then incubated with 20 μl of each stimulant in a 96-well plate. After 20 h of culture, the optical density at 630 nm (OD630) was measured.
Flow cytometry
Single lung cell suspensions were blocked using anti-mouse CD16/CD32 (mouse BD Fc Block™; BD Biosciences) at room temperature for 15 min before staining. The surface antigens were stained with the indicated conjugated antibodies at 4 °C for 15 min. The following antibodies were used: APC anti-CD4 (Thermo Fisher Scientific, Cat# 17-0042-82), PerCP-eFluor 710 anti-CD3e (Thermo Fisher Scientific, Cat# 46-0033-82), PE/Cyanine7 anti-CD45R (Thermo Fisher Scientific, Cat# 25-0452-82), PE anti-Siglec-F (Thermo Fisher Scientific, Cat# 552126), PerCD-eFluor 710 anti-Ly6G (Thermo Fisher Scientific, Cat# 46-9668-82), Alexa Fluor 700 anti-MHC Class II I-A/I-E (Thermo Fisher Scientific, Cat# 56-5321-80), and eFluor 450 anti-F4/80 (Thermo Fisher Scientific, Cat# 48-4801-82); APC/cyanine7 anti-CD45 (BioLegend, San Diego, CA, USA, Cat# 103116); APC anti-CD11b (BioLegend, Cat# 101212) and PE anti-CD49b (BioLegend, Cat# 103506); and FITC anti-NK1.1 (BD Biosciences, Cat# 553164). Stained cells were analyzed using a Gallios flow cytometer (Beckman Coulter, Brea, CA, US) with FlowJo™ software (Becton, Dickinson and Company, New Jersey, USA).
Statistical analysis
Statistical differences among groups were assessed using a two-tailed Student’s t-test and a log-rank test with Prism software (GraphPad Software, USA). Mice were randomly assigned to experimental groups without blinding method for injections. No animal exclusion criteria were applied, and the variance was comparable among the groups that were statistically compared. Values with p < 0.05 were considered to be statistically significant.
Data availability
All datasets generated and analysed during this study are included in this published article and its Supplementary Information files. Additional data are available from the corresponding author on reasonable request.
References
Julkunen I, Melen K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19:S32–37.
Lee SM, Cheung CY, Nicholls JM, Hui KP, Leung CY, Uiprasertkul M, et al. Hyperinduction of cyclooxygenase-2-mediated proinflammatory cascade: a mechanism for the pathogenesis of avian influenza H5N1 infection. J Infect Dis. 2008;198:525–35.
Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–95.
Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–12.
Chang P, Kuchipudi SV, Mellits KH, Sebastian S, James J, Liu J, et al. Early apoptosis of porcine alveolar macrophages limits avian influenza virus replication and pro-inflammatory dysregulation. Sci Rep. 2015;5:17999.
Lam WY, Tang JW, Yeung AC, Chiu LC, Sung JJ, Chan PK. Avian influenza virus A/HK/483/97(H5N1) NS1 protein induces apoptosis in human airway epithelial cells. J Virol. 2008;82:2741–51.
Hartmann BM, Albrecht RA, Zaslavsky E, Nudelman G, Pincas H, Marjanovic N, et al. Pandemic H1N1 influenza A viruses suppress immunogenic RIPK3-driven dendritic cell death. Nat Commun. 2017;8:1931.
Kim C-U, Jeong Y-J, Lee P, Lee M-S, Park J-H, Kim Y-S, et al. Extracellular nucleoprotein exacerbates influenza virus pathogenesis by activating Toll-like receptor 4 and the NLRP3 inflammasome. Cell Mol Immunolog. 2022;19:715–25.
Peukes J, **ong X, Erlendsson S, Qu K, Wan W, Calder LJ, et al. The native structure of the assembled matrix protein 1 of influenza A virus. Nature. 2020;587:495–8.
Halder UC, Bagchi P, Chattopadhyay S, Dutta D, Chawla-Sarkar M. Cell death regulation during influenza A virus infection by matrix (M1) protein: a model of viral control over the cellular survival pathway. Cell Death Dis. 2011;2:e197.
Zhirnov OP, Ksenofontov AL, Kuzmina SG, Klenk HD. Interaction of influenza A virus M1 matrix protein with caspases. Biochemistry (Mosc). 2002;67:534–9.
Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–49.
Pone EJ, Hernandez-Davies JE, Jan S, Silzel E, Felgner PL, Davies DH. Multimericity amplifies the synergy of BCR and TLR4 for B cell activation and antibody class switching. Front Immunol. 2022;13:882502.
Selzer L, Su Z, Pintilie GD, Chiu W, Kirkegaard K. Full-length three-dimensional structure of the influenza A virus M1 protein and its organization into a matrix layer. PLoS Biol. 2020;18:e3000827.
Zhao H, Ekstrom M, Garoff H. The M1 and NP proteins of influenza A virus form homo- but not heterooligomeric complexes when coexpressed in BHK-21 cells. J Gen Virol. 1998;79:2435–46.
Chen H, Ning X, Jiang Z. Caspases control antiviral innate immunity. Cell Mol Immunol. 2017;14:736–47.
Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–92.
Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–77.
Hogner K, Wolff T, Pleschka S, Plog S, Gruber AD, Kalinke U, et al. Macrophage-expressed IFN-beta contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 2013;9:e1003188.
Rodrigue-Gervais IG, Labbé K, Dagenais M, Dupaul-Chicoine J, Champagne C, Morizot A, et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe. 2014;15:23–35.
Mosavi SZ, Shahsavandi S, Ebrahimi MM, Hatami AR, Sadeghi K, Shahivandi H. Necrotic response to low pathogenic H9N2 influenza virus in chicken hepatoma cells. Jundishapur J Microbiol. 2015;8:e13770.
Arndt U, Wennemuth G, Barth P, Nain M, Al-Abed Y, Meinhardt A, et al. Release of macrophage migration inhibitory factor and CXCL8/interleukin-8 from lung epithelial cells rendered necrotic by influenza A virus infection. J Virol. 2002;76:9298–306.
Balachandran S, Rall GF. Benefits and perils of necroptosis in influenza virus infection. J Virol. 2020;94:e01101–19.
Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010;17:922–30.
Hofer CT, Di Lella S, Dahmani I, Jungnick N, Bordag N, Bobone S, et al. Structural determinants of the interaction between influenza A virus matrix protein M1 and lipid membranes. Biochim Biophys Acta Biomembr. 2019;1861:1123–34.
Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–91.
Koyama S, Aoshi T, Tanimoto T, Kumagai Y, Kobiyama K, Tougan T, et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med. 2010;2:25ra24.
Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–20.
Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol. 2004;173:6882–9.
Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–65.
Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87.
Thomas PG, Dash P, Aldridge JR Jr., Ellebedy AH, Reynolds C, Funk AJ, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–75.
Uiprasertkul M, Kitphati R, Puthavathana P, Kriwong R, Kongchanagul A, Ungchusak K, et al. Apoptosis and pathogenesis of avian influenza A (H5N1) virus in humans. Emerg Infect Dis. 2007;13:708–12.
Chen KK, Minakuchi M, Wuputra K, Ku CC, Pan JB, Kuo KK, et al. Redox control in the pathophysiology of influenza virus infection. BMC Microbiol. 2020;20:214.
Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–22.
Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–8.
Oberkampf M, Guillerey C, Mouries J, Rosenbaum P, Fayolle C, Bobard A, et al. Mitochondrial reactive oxygen species regulate the induction of CD8(+) T cells by plasmacytoid dendritic cells. Nat Commun. 2018;9:2241.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Molteni M, Gemma S, Rossetti C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediators Inflamm. 2016;2016:6978936.
Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32.
Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–4.
Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, Kaech SM. TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T Cell differentiation. J Immunol. 2014;192:4221–32.
Luan L, Patil NK, Guo Y, Hernandez A, Bohannon JK, Fensterheim BA, et al. Comparative transcriptome profiles of human blood in response to the toll-like receptor 4 ligands lipopolysaccharide and monophosphoryl lipid A. Sci Rep. 2017;7:40050.
Padilla-Quirarte HO, Lopez-Guerrero DV, Gutierrez-**cotencatl L, Esquivel-Guadarrama F. Protective antibodies against influenza proteins. Front Immunol. 2019;10:1677.
Vanderven HA, Ana-Sosa-Batiz F, Jegaskanda S, Rockman S, Laurie K, Barr I, et al. What lies beneath: antibody dependent natural killer cell activation by antibodies to internal influenza virus proteins. EBioMedicine. 2016;8:277–90.
Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol. 2008;181:4168–76.
LaMere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. 2011;186:4331–9.
Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, et al. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with mycobacterium tuberculosis. Gene Ther. 2003;10:1592–9.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grants funded by the Government of Korea (MSIT: 2018M3A9H4077992 and 2020R1C1C1008451) and the KRIBB Research Initiative Program (KGM9952112 and KGM9942213).
Funding
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Government of Korea (MSIT: 2018M3A9H4077992 and 2020R1C1C1008451) and the KRIBB Research Initiative Program (KGM9942213 and KGM9952112).
Author information
Authors and Affiliations
Contributions
Study conception and design: CUK, DL, YSK, DJK. Methodology: CUK, DJK. Analysis and interpretation of results: CUK, DL, BK, DJK. Investigation: CUK, DL, BK, YSK, DJK. Validation: CUK, DL, YSK, BK, DJK. Resources: BK, DJK. Original draft preparation: CUK, DL, BK. Draft review & editing: CUK, BK, DJK. Final edition: BK, DJK. Supervision: BK, DJK. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Hans-Uwe Simon
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, CU., Lim, D., Kim, Y.S. et al. Influenza viral matrix 1 protein aggravates viral pathogenicity by inducing TLR4-mediated reactive oxygen species production and apoptotic cell death. Cell Death Dis 14, 228 (2023). https://doi.org/10.1038/s41419-023-05749-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-05749-5
- Springer Nature Limited