Abstract
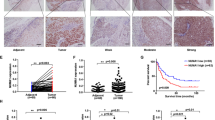
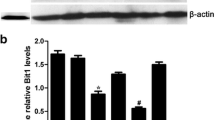
TAB182 (also named TNKS1BP1), a binding protein of tankyrase 1, has been found to participate in DNA repair. Our previous study has revealed the involvement of TAB182 in the radioresistance of esophageal squamous cell carcinoma (ESCC) cells. However, whether TAB182 contributes to the ESCC tumorigenesis and progression remains unclear. In this study, we found that highly expressed TAB182 is closely associated with a poor prognosis of patients with ESCC. TAB182 silencing reduced ESCC cell proliferation and invasion in vitro, tumorigenicity and metastasis in vivo. RNA-seq and IP-MS analysis revealed that TAB182 could affect the β-catenin signaling pathway via interacting with β-catenin. Furthermore, TAB182 prevented β-catenin to be phosphorylated by GSK3β and recruited four and a half of LIM-only protein 2 (FHL2), which thereby promoted β-catenin nucleus translocation to result in activation of the downstream targets transcription in ESCC cells. Our findings demonstrate that TAB182 enhances tumorigenesis of esophageal cancer by promoting the activation of the β-catenin signaling pathway, which provides new insights into the molecular mechanisms by which TAB182 accelerates progression of ESCC.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is the sixth most prevalently diagnosed malignancy in the world with estimated 450,000 deaths every year. Strikingly, China accounts for half of the global morbidity as well as mortality of EC [1, 2]. Esophageal squamous cell carcinoma (ESCC), as well as esophageal adenocarcinoma (EAC), is the primary histological types of EC. In China, ESCC is the most predominant subtype with high incidences, accounting for 90% of newly diagnosed EC cases [3, 6]. Recently, Zhong et al. reported that TAB182 is up-regulated in lung adenocarcinoma and affects lung adenocarcinoma cells’ sensitivity to DNA damage regent through regulating the homologous recombination pathway of DNA double-strand breaks (DSB) [7]. Moreover, Zhou and colleagues found that TAB182 could be induced by ionizing radiation (IR), and TAB182 is required for the efficient repair of IR-mediated DSB through enhancing DNA-PKcs’ interacting with PARP-1 [8]. Our previous study showed that TAB182 heightens the radioresistance of ESCC cells by mediating the G2-M checkpoint via its interaction with FHL2 [9]. Although recent studies have revealed the crucial roles of TAB182 in DNA damage response, whether and how TAB182 associates with ESCC tumorigenesis and progression remain unclear.
FHL2 is a multifunctional scaffolding protein that belongs to the four-and-a-half LIM domains protein family. FHL2 has been found to interact with multiple types of proteins due to its unique structure that consists of several LIM motifs, including IER3 and MDM2 [10], EGFR, and EGFRvIII [11]. Additionally, it is reported that the zinc-finger motif in the LIM domain confers FHL2 with transcriptional repressor or co-activator function [12, 13]. As a result, FHL2 is involved in the modulation of varied cellular processes, including gene expression, cell proliferation and motility [14]. Interestingly, accumulating evidence has demonstrated the critical roles of FHL2 in tumorigenesis and the progression of carcinoma in a context-dependent manner. For instance, it exerts oncogenic function in ovarian cancer [15], cervical cancer [10], and gastrointestinal cancer [28], suggesting that TAB182 may act differently in different types of cancer cells.
Tumorigenesis and tumor malignant nature are often associated with cancer cell stemness. ESCC cells with cancer stem cells characteristics have been considered to more inclined to distant metastasis, resulting in poor prognosis of the patients [29]. Here, we discovered that TAB182 promotes the stemness and tumorigenicity of ESCC cells by triggering the nucleus translocation of β-catenin to stimulate the β-catenin pathway, which leads to the activated transcription of JUN, MYC, ALDH1A1, CD44, and CD133. In accordance with our findings, Huang revealed that the activation of β-catenin signaling enhances the androgen-independent self-renewal of ESCC cells with stem cell-like characteristics [30]. While the molecular processes underlying the modulation of β-catenin by TAB182 in ESCC remain to be further elucidated, we discovered that TAB182 physically interacts with β-catenin and prevents it from being phosphorylated by GSK3β and ubiquitination-mediated degradation. TAB182 further recruits FHL2 and forms a TAB182-FHL2-β-catenin complex allowing for efficient FHL2-mediated β-catenin nucleus translocation by enhancing the association between FHL2 and β-catenin, and this is dependent on the RXXPDG motif of TAB182 in ESCC cells. These findings indicated that TAB182 may serve as a crucial malignant factor and novel stemness-related modulator via the TAB182-FHL2-β-catenin axis in ESCC.
Conclusions
In summary, our study reveals the novel oncogenic role of TAB182 in ESCC and avail useful insights into a probable function of the TAB182/FHL2/β-catenin molecular axis in ESCC cell stemness, invasiveness, and tumorigenicity. Of clinical relevance, TAB182 might function as a potential diagnostic marker for ESCC. Additionally, targeting the TAB182/FHL2/β-catenin can be a new avenue for the development of therapeutics against ESCC.
Data availability
The dataset(s) supporting the findings of this study are included within the article.
Materials availability
Requests for materials should be addressed to J-DZ and M-S.
Change history
14 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41419-023-05799-9
References
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: a cancer J clinicians. 2016;66:115–32.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Hou H, Meng Z, Zhao X, Ding G, Sun M, Wang W, et al. Survival of Esophageal Cancer in China: A Pooled Analysis on Hospital-Based Studies From 2000 to 2018. Front Oncol. 2019;9:548.
Shen Y, **e S, Zhao L, Song G, Shao Y, Hao C, et al. Estimating Individualized Absolute Risk for Esophageal Squamous Cell Carcinoma: A Population-Based Study in High-Risk Areas of China. Front Oncol. 2020;10:598603.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Seimiya H, Smith S. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182). J Biol Chem. 2002;277:14116–26.
Tan W, Guan H, Zou LH, Wang Y, Liu XD, Rang WQ, et al. Overexpression of TNKS1BP1 in lung cancers and its involvement in homologous recombination pathway of DNA double-strand breaks. Cancer Med. 2017;6:483–93.
Zou LH, Shang ZF, Tan W, Liu XD, Xu QZ, Song M, et al. TNKS1BP1 functions in DNA double-strand break repair though facilitating DNA-PKcs autophosphorylation dependent on PARP-1. Oncotarget. 2015;6:7011–22.
Cao Y, Gao A, Li X, Min H, He C, Sun X, et al. Elevated TAB182 enhances the radioresistance of esophageal squamous cell carcinoma through G2-M checkpoint modulation. Cancer Med. 2021;10:3101–12.
** H, Lee K, Kim YH, Oh HK, Maeng YI, Kim TH, et al. Scaffold protein FHL2 facilitates MDM2-mediated degradation of IER3 to regulate proliferation of cervical cancer cells. Oncogene. 2016;35:5106–18.
Sun L, Yu S, Xu H, Zheng Y, Lin J, Wu M, et al. FHL2 interacts with EGFR to promote glioblastoma growth. Oncogene. 2018;37:1386–98.
Verset L, Feys L, Trépant AL, De Wever O, Demetter P. FHL2: a scaffold protein of carcinogenesis, tumour-stroma interactions and treatment response. Histol Histopathol. 2016;31:469–78.
Cao CY, Mok SW, Cheng VW, Tsui SK. The FHL2 regulation in the transcriptional circuitry of human cancers. Gene. 2015;572:1–7.
Johannessen M, Moller S, Hansen T, Moens U, Van, Ghelue M. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci. 2006;63:268–84.
Hua G, He C, Lv X, Fan L, Wang C, Remmenga SW, et al. The four and a half LIM domains 2 (FHL2) regulates ovarian granulosa cell tumor progression via controlling AKT1 transcription. Cell death Dis. 2016;7:e2297.
Wang J, Yang Y, **a HH, Gu Q, Lin MC, Jiang B, et al. Suppression of FHL2 expression induces cell differentiation and inhibits gastric and colon carcinogenesis. Gastroenterology. 2007;132:1066–76.
Paul C, Lacroix M, Iankova I, Julien E, Schafer BW, Labalette C, et al. The LIM-only protein FHL2 is a negative regulator of E4F1. Oncogene. 2006;25:5475–84.
Ng CF, Xu JY, Li MS, Tsui SK. Identification of FHL2-regulated genes in liver by microarray and bioinformatics analysis. J Cell Biochem. 2014;115:744–53.
Zou S, Shang ZF, Liu B, Zhang S, Wu J, Huang M, et al. DNA polymerase iota (Pol iota) promotes invasion and metastasis of esophageal squamous cell carcinoma. Oncotarget. 2016;7:32274–85.
He C, Wu S, Gao A, Su Y, Min H, Shang ZF, et al. Phosphorylation of ETS-1 is a critical event in DNA polymerase iota-induced invasion and metastasis of esophageal squamous cell carcinoma. Cancer Sci. 2017;108:2503–10.
Cai T, Sun D, Duan Y, Qiu Y, Dai C, Yang J, et al. FHL2 promotes tubular epithelial-to-mesenchymal transition through modulating beta-catenin signalling. J Cell Mol Med. 2018;22:1684–95.
Sbodio JI, Chi NW. Identification of a tankyrase-binding motif shared by IRAP, TAB182, and human TRF1 but not mouse TRF1. NuMA contains this RXXPDG motif and is a novel tankyrase partner. J Biol Chem. 2002;277:31887–92.
Wang W, Shao F, Yang X, Wang J, Zhu R, Yang Y, et al. METTL3 promotes tumour development by decreasing APC expression mediated by APC mRNA N(6)-methyladenosine-dependent YTHDF binding. Nat Commun. 2021;12:3803.
de Castro J, Gamallo C, Palacios J, Moreno-Bueno G, Rodríguez N, Feliu J, et al. beta-catenin expression pattern in primary oesophageal squamous cell carcinoma. Relationship with clinicopathologic features and clinical outcome. Virchows Arch: Int J Pathol. 2000;437:599–604.
Ren HZ, Wang JS, Pan GQ, Lv H, Wen JF, Luo GQ, et al. Comparative proteomic analysis of beta-catenin-mediated malignant progression of esophageal squamous cell carcinoma. Dis Esophagus: Off J Int Soc Dis Esophagus. 2010;23:175–84.
Hiremath IS, Goel A, Warrier S, Kumar AP, Sethi G, Garg M. The multidimensional role of the Wnt/beta-catenin signaling pathway in human malignancies. J Cell Physiol. 2021;237:199–238.
Chatterjee A, Paul S, Bisht B, Bhattacharya S, Sivasubramaniam S, Paul MK. Advances in targeting the WNT/beta-catenin signaling pathway in cancer. Drug Discov Today. 2021;27:82–101.
Ohishi T, Yoshida H, Katori M, Migita T, Muramatsu Y, Miyake M, et al. Tankyrase-Binding Protein TNKS1BP1 Regulates Actin Cytoskeleton Rearrangement and Cancer Cell Invasion. Cancer Res. 2017;77:2328–38.
Li MY, Fan LN, Han DH, Yu Z, Ma J, Liu YX, et al. Ribosomal S6 protein kinase 4 promotes radioresistance in esophageal squamous cell carcinoma. J Clin Investig. 2020;130:4301–19.
Huang D, Gao Q, Guo L, Zhang C, Jiang W, Li H, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem cells Dev. 2009;18:465–73.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China, Grant/Award Number: 81672975 and 81802341; Natural Science Foundation of Jiangsu Province (BK20220259); Suzhou Administration of Science & Technology, Grant/Award Number: SS2019013 and SYS2019091; Suzhou Key Medical Center, Grant/Award Number: SZZX201506; The Affiliated Suzhou Hospital of Nan**g Medical University, Suzhou Municipal Hospital, Gusu School, Grant/Award Number: GSKY202110211.
Author information
Authors and Affiliations
Contributions
YZ, MS and JDZ designed and supervised the study, ADG and ZZS conducted the experiment. ZFS, CH and DLM contributed to acquisition of results. LXQ, STZ, WQD and HC performed the data analysis. ADG and MS wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The procedures of this study were approved by the Research Ethics Committee of The Affiliated Suzhou Hospital of Nan**g Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Nickolai Barlev
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, A., Su, Z., Shang, Z. et al. TAB182 aggravates progression of esophageal squamous cell carcinoma by enhancing β-catenin nuclear translocation through FHL2 dependent manner. Cell Death Dis 13, 900 (2022). https://doi.org/10.1038/s41419-022-05334-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-05334-2
- Springer Nature Limited