Abstract
Transforming growth factor (TGF)-β1, a main profibrogenic cytokine in the progression of idiopathic pulmonary fibrosis (IPF), induces differentiation of pulmonary fibroblasts to myofibroblasts that produce high levels of collagen, leading to concomitantly loss of lung elasticity and function. Recent studies implicate the importance of microRNAs (miRNAs) in IPF but their regulation and individual pathological roles remain largely unknown. We used both RNA sequencing and quantitative RT-PCR strategies to systematically study TGF-β1-induced alternations of miRNAs in human lung fibroblasts (HFL). Our data show that miR-133a was significantly upregulated by TGF-β1 in a time- and concentration-dependent manner. Surprisingly, miR-133a inhibits TGF-β1-induced myofibroblast differentiation whereas miR-133a inhibitor enhances TGF-β1-induced myofibroblast differentiation. Interestingly, quantitative proteomics analysis indicates that miR-133a attenuates myofibroblast differentiation via targeting multiple components of TGF-β1 profibrogenic pathways. Western blot analysis confirmed that miR-133a down-regulates TGF-β1-induced expression of classic myofibroblast differentiation markers such as ɑ-smooth muscle actin (ɑ-SMA), connective tissue growth factor (CTGF) and collagens. miRNA Target Searcher analysis and luciferase reporter assays indicate that TGF-β receptor 1, CTGF and collagen type 1-alpha1 (Col1a1) are direct targets of miR-133a. More importantly, miR-133a gene transferred into lung tissues ameliorated bleomycin-induced pulmonary fibrosis in mice. Together, our study identified TGF-β1-induced miR-133a as an anti-fibrotic factor. It functions as a feed-back negative regulator of TGF-β1 profibrogenic pathways. Thus, manipulations of miR-133a expression may provide a new therapeutic strategy to halt and perhaps even partially reverse the progression of IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and fatal disease associated with deteriorating lung functioning, characterized by an excessive accumulation of extracellular matrix (ECM) proteins in response to chronic lung injury1. For patients with IPF, median survival time is 2–3 years from diagnosis. The pathological hallmarks of IPF include recruitment of inflammatory cells and excessive secretion of profibrotic cytokines such as transforming growth factor-β (TGF-β), aberrant fibroblast differentiation and proliferation, and persistence of apoptotic resistant myofibroblasts in the lesions2,3. TGF-β plays a central role in the pathogenesis of pulmonary fibrosis by promoting differentiation of fibroblasts into myofibroblasts that produce excessive extracellular matrix resulting in deteriorating lung function4,5,6,7. Treatment of fibroblasts with TGF-β leads to the phosphorylation and activation of Smad transcriptional factors that in turn result in the activation or repression of target genes including the differentiation marker genes α-smooth muscle actin (α-SMA), connective tissue growth factor (CTGF) and collagens5. In addition to this canonical Smad signaling pathway, non-canonical p38 mitogen-activated protein kinase (p38MAPK) signaling pathways also contribute to TGF-β-induced fibroblast differentiation6,7. Non-canonical signaling is context dependent and may have different effects in various cell types5,8.
MiRNAs emerged as a class of small RNAs that regulate whole networks of genes during different biological processes9. Several miRNAs such as miR-21, miR-218 and miR-29 have been shown to be dysregulated in fibrotic conditions10,11,12, and alterations in miRNA expression patterns may contribute to fibroblast differentiation4e, f).
a–d Heatmap representing the log2 (abundances) of the differentially expressed genes in the selected KEGG pathways in miR-133a overexpressed HFL cells as compared to the CTL groups. The color key from blue to red represents the log2 (abundances) from low to high. e The overlap between Targetscan predicted miR-133a targets and miR-133a-down-regulated proteins identified by quantitative proteomics. f 22 overlap** proteins were analyzed and shown as Heatmap clusters
Overexpression of miR-133a alters the phenotype of TGF-β1-treated HFL cells
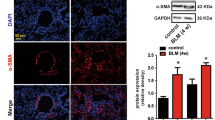
Quantitative proteomic results and Targetscan prediction suggested that overexpression of miR-133a in myofibroblasts strongly perturbs the transcriptome, particularly myofibroblast markers. Indeed, quantitative RT-PCR analysis indicated that treatment with TGF-β1 for 48 h significantly increased mRNA expression levels of Col1a1 and α-SMA (ACTA2) (Fig. 5a). Cells were further treated with TGF-β1 for an additional 48 h in the presence of miR-133a mimic or control mimic. Compared to control mimic, miR-133a mimic markedly reduced mRNA expression levels of Col1a1 and α-SMA by 77 and 72%, respectively, which is much lower than that of cells prior to this additional 48 h treatment with TGF-β1 (Fig. 5a). Immunostaining of α-SMA also showed a decrease of stress fiber formation after miR-133a overexpression in myofibroblasts (Fig. 5b).
HFL cells were treated with 1 ng/mL of TGF-β1 for 48 h, and then transfected with miR-133a mimic or its control (CTL) for an additional 48 h. a Total RNAs were harvested and subjected to quantitative RT-PCR analysis of Col1a1 and ACTA2 mRNA expression. Data are mean ± SEM (n = 3) with ***P < 0.001. b HFL cell differentiation was assessed by immunofluorescence staining for α-SMA. Left: representative staining images of α-SMA–positive stress fibers (green) and DAPI (blue) showing nuclei under confocal laser scanning microscopy. Right: bright field image and DAPI staining of nuclei (blue) (scale bar = 30 μm). c Quantitative RT-PCR analysis of MMP-2 and MMP-9 mRNA expression. d Culture media were collected and run on 10% SDS-PAGE contains 1% collagen. Gels were incubated with MMP activity testing buffer for a gelatin zymography assay and then stained with Coomassie blue (left). Total proteins harvested from whole cell lysis run on 10% SDS were used as internal controls (right)
ECM protein deposition in the lung is the primary cause of death of pulmonary fibrosis patients20. Matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9) are secreted proteins involved in the breakdown of ECM proteins. Quantitative RT-PCR analysis showed that MMP-2 but not MMP-9 was induced by TGF-β1 treatment, which was not affected by overexpression of miR-133a (Fig. 5c). Gelatin zymography confirmed the unchanged activities of MMP-2 and MMP-9 in cells transfected with miR-133a mimic as compared to cells with control mimic (Fig. 5d). Thus, overexpression of miR-133a reduces the expression of ECM proteins without effects on expression and activity of matrix metalloproteinases.
MiR-133a directly targets multiple components of TGF-β1 signaling pathways
A total of 605 putative target genes were identified by using miRNA Target Searcher (http://www.targetscan.org/) (Supplementary Table 1), well-established target prediction algorithm for miRNAs. Further KEGG pathway analysis revealed several components of TGF-β signaling pathways such as TGFBR1, CTGF, and Col1A1 as putative miR-133a targets (Fig. 6a). Indeed, miR-133a mimic remarkably down-regulated TGFBR1 protein expression in HFL cells (Fig. 6b). Consequently, TGF-β1-induced phosphorylation of Smad2/3 was inhibited by miR-133a mimic (Fig. 6c). Similarly, overexpression of miR-133a also resulted in a significant decrease in TGF-β1-induced expression of CTGF protein and Col1a1 mRNA, respectively (Fig. 6d, e). Interestingly, TGF-β1-induced expression of Col4a1, not a predicted target of miR-133a, was also significantly reduced by miR-133a (Fig. 6f).
a KEGG analysis of Targetscan predicted miR-133a targets. HFL cells were transfected with miR-133a mimic or control mimic (CTL) for 24 h, and then stimulated without or with 1 ng/mL TGF-β1 for 48 h. Cells were harvested and subjected to western blot (b–d) or quantitative RT-PCR analysis (e, f). Transfection of miR-133a down-regulated TGFBR1 protein expression (b), blocked TGF-β1-stimulated Smad2/3 phosphorylation (c), CTGF protein expression (d), Col1a1 (e), and Col4a1 (f) mRNA expression. Data are mean ± SEM (n = 3–4) with *P < 0.05; ***P < 0.001. g The 3′UTR of TGFBR1, CTGF or Col1a1 contains a putative miR-133a binding site that is conserved among different species (in red). miR-133a binding site mutations were created by deletion or nucleotide changes (in blue). h The 3′UTR fragments containing the putative miR-133a binding site (3’UTR-133a) or their mutants (3′UTR-133aM) of human TGFBR1 (inserted fragment: 1989–2403; seeding region: 2161–2167), CTGF (inserted fragment: 886–1065; seeding region: 1027–1033), or Col1a1 (inserted fragment 78–301; seeding region: 194–200) were subcloned into the pmirGLO reporter vector. i These luciferase reporter constructs were transfected into HEK293 cells, along with control or miR-133a mimic. Cells were harvested for firefly luciferase (fLuc) assays with Renilla luciferase (rLuc) as an internal control. Data are mean ± SEM (n = 3–4) with **P < 0.01
We further examined alterations of TGF-β1 signaling pathways by miR-1, a member of the miR-1/miR-133a cluster21 that was also upregulated in TGF-β1-treated HFL cells (Table 1 and Fig. 1a). As shown in Supplementary Fig. 3, compared to control mimic, transfection of miR-1 mimic did not change TGFBR1 protein expression or TGF-β1-induced upregulation of CTGF protein or Col1a1 and Col4a1 mRNAs. This is consistent with data showing no effects of miR-1 on TGF-β1-induced α-SMA expression (Fig. 1b). Thus, miR-133a but not miR-1 specifically targets TGF-β1 profibrogenic signaling pathways to inhibit pulmonary fibroblast differentiation.
Alignment of the 3’UTR of TGFBR1, CTGF and Col1a1 among a wide range of species (mouse, rabbit and human) using the Targetscan bioinformatic tool revealed that the predicted binding sites for miR-133a are highly conserved during evolution (Fig. 6g). To determine whether TGFBR1, CTGF and Col1a1 are direct targets of miR-133a, we inserted the fragment of TGFBR1 3′UTR, CTGF 3′UTR or Col1a1 3′UTR containing the miR-133a putative target site (UTR-133a) or their mutants at the seeding region (UTR-133a/M) into the pmirGLO dual-luciferase reporter vector (Fig. 6h). Compared to control mimic, miR-133a mimic reduced the luciferase activity by about 35% (**P < 0.01), which was completely abolished by mutations at their seeding regions (Fig. 6i). These data suggest a novel function of TGF-β1-induced miR-133a as a negative feedback regulator of TGF-β1 profibrogenic signaling pathways in pulmonary fibroblasts via directly repressing TGFBR1, CTGF and collagen expression.
MiR-133a gene transferred into lung tissues ameliorates bleomycin-induced pulmonary fibrosis in mice
The mouse model of bleomycin-induced lung fibrosis is widely used to explore both the pathogenesis of IPF and potential new therapies22. We used this mouse model to investigate whether overexpression of miR-133a in vivo has therapeutic benefits. We first constructed a miR-133a expression plasmid pAAV-FTS1-GFP-miR-133a and its control pAAV-FTS1-GFP vector that contains the fibroblast-specific protein-1 (FSP1) gene promoter (Fig. 7a). In vitro transfection of this pAAV-FTS1-GFP plasmid into 16HBE epithelial cells and NIH3T3 fibroblast cells showed selective expression of GFP in fibroblasts. In contrast, the plasmid pAAV-CMV-GFP with the CMV promoter had no such selectivity (Fig. 7b). Quantitative RT-PCR confirmed the selective expression of miR-133a in fibroblast cells by the pAAV-FTS1-GFP-133a plasmid (Fig. 7c). Mice were intratracheally administered bleomycin on day 0. After 5 days, the pAAV-FTS1-GFP-miR-133a or pAAV-FTS1-GFP control vector complexed with the EntransterTM-in vivo agent was delivered into mice via tail vein injection and this procedure was repeated every 4 days. On day 15, mouse lung tissues were collected and subjected to histochemical analysis (Fig. 7d). H&E and trichrome staining indicated that bleomycin exposure induced collagen deposition in lungs of mice and that administration of the miR-133a vector, but not the control vector, ameliorated bleomycin-induced fibrotic lung lesions in mice (Fig. 7e). Scoring the bleomycin-induced histological fibrosis by the Ashcroft method confirmed pulmonary fibrosis in bleomycin-treated mice as compared with the sham group (6.3 ± 0.3 vs. 1.2 ± 0.1, ***P < 0.001) and administration of the vector expressing miR-133a significantly reduced the Ashcroft score of bleomycin-treated mice from 6.1 ± 0.1 to 4.2 ± 0.1 (Fig. 7f, ***P < 0.001).
a The cis-element of miR-133a expression vector driven by the FTS1 promoter. b Confocal laser scanning microscopy images of GFP expressed in NIH3T3 fibroblasts or epithelial 16HBE cells using pAAV-CMV-GFP or pAAV-FTS1-GFP vector. Scale bar: 50 μm. c pAAV-CMV-GFP-133a or pAAV-FTS1-GFP-133a vector was transfected into NIH3T3 fibroblasts or epithelial 16HBE cells. 24 h later, the expression levels of miR-133a were determined by quantitative RT-PCR. d Experimental scheme of the mouse model of bleomycin-induced pulmonary fibrosis. Mice were intratracheally injected with saline or bleomycin (50 mg/kg) at day 0. On day 5, mice were administrated pAAV-FTS1-GFP-miR-133a expression vector or its control vector by tail vein injection. This treatment was repeated every 4 days and mice were killed on day 15. e Photomicrographs showing H&E staining and Masson staining of lung tissues of mice treated with saline, bleomycin without or with miR-133a expression vector or its control vector. Scale bar: 200 μm. f Standardized quantification of pulmonary fibrosis in Masson staining samples by the modified Ashcroft method (score range 0–8). Data are mean ± SEM, n = 4, ***P < 0.001. g Graphical summary of results. TGF-β1-induced miR-133a functions as a feed-back negative regulator of TGF-β1 profibrogenic pathways. TGF-β1 induces miR-133a expression via Smad3 and p38MAPK signaling pathways. Upregulated miR-133a in turn negatively regulates myofibroblast differentiation via targeting multiple components of the TGF-β1 signal pathway such as TGFBR1, CTGF, and Col1a1
Discussion
Pulmonary fibrosis is characterized by increased pulmonary myofibroblasts with lung stiffening due to an accumulation of extracellular matrix and loss of alveolar cells23. Cytokine TGF-β has long been recognized as a major driver of lung fibrosis by promoting differentiation of fibroblasts into myofibroblasts. Surprisingly, our current study identified TGF-β1-induced miR-133a as an anti-fibrotic factor. It functions as a negative feedback regulator of profibrogenic pathways by targeting a panel of TGF-β1 signaling pathway related proteins in human pulmonary fibroblasts.
MicroRNAs are now recognized as important regulators of cellular function in both health and disease9,24,25, and are capable of exerting effects both in the cells in which they are synthesized and in other cells via uptake of circulating miRNAs secreted into exosomes26. Since TGF-β plays a central role in pulmonary fibrosis by promoting differentiation of fibroblast cells into myofibroblast cells, we performed RNA-sequencing to identify differentially expressed miRNAs in HFL cells with or without TGF-β1 treatment. Our initial hypothesis was that miRNAs upregulated by TGF-β1 may mediate TGF-β1 profibrogenic signaling to promote pulmonary fibrosis. Considering time, cost and other factors, we chose the miR-1/miR-133a cluster for further investigation since it has not been reported to be related to pulmonary fibrosis. We also chose the miR-143/miR-145 cluster and miR-21 as positive controls since these miRNAs were among the TGF-β1-induced miRNAs that promote myofibroblast differentiation and fibrosis14,27. Among these five selected miRNAs, transfection of miR-143 or miR-145 enhanced TGF-β1-induced α-SMA expression. Surprisingly, transfection of miR-133a, but not miR-1, another member of the miR-1/miR-133a cluster, inhibited TGF-β1-induced α-SMA expression, suggesting that miR-133a may specifically function as an anti-fibrotic factor.
MiR-133a is among the better studied microRNAs and has been implicated in cancer development, skeletal muscle cell differentiation, as well as in cardiac fibrosis21,28,29,30. Several studies have implicated dysregulation of miR-133a in lung diseases. For example, decreases in miR-133a have been linked to increases in RhoA pathway activity in bronchiolar smooth muscle and airway hyperresponsiveness due to IL-13 or IL-17 treatment31,32. However, miR-133a clearly has a broader and more nuanced role in the lungs. We recently identified that increase in miR-133a in airway epithelial cells, whether triggered by exposure to TGF-β or cigarette smoke, is a crucial factor in their epithelial-to-mesenchymal transition in association with increased airway reactivity to methacholine33.
The present study has identified an additional role of miR-133a in human pulmonary fibroblasts whose differentiation are regulated by miR-133a. Our data show that in a pre-defined set of miRNAs relevant to pulmonary pathology, miR-133a levels are elevated in differentiated myofibroblasts induced by TGF-β1, and correlate with markers of pulmonary fibrosis. This upregulation of miR-133a by TGF-β1 is time and concentration dependent and was not replicated by another cytokine TNF-α also released by cells during wound healing. TGF-β1-induced miR-133a upregulation was attenuated by inhibitors of Smad3 and p38-MAPK signaling pathways, suggesting that miR-133a may serve as a feedback mediator down-regulating profibrotic genes and desensitizing TGF-β1 signaling pathways. Indeed, TGF-β1 induced expression of α-SMA in these fibroblasts was inhibited by miR-133a mimic while α-SMA expression was enhanced by addition of miR-133a inhibitor. Quantitative proteomics analysis indicated that miR-133a inhibits ECM synthesis and cell motility-related genes in TGF-β1-treated fibroblasts. These findings are in accordance with our RT-PCR and western blot data showing that miR-133a down-regulates the RNAs/proteins of Col1a1, Col4a1 and α-SMA in TGF-β1-treated cells. Interestingly, miR-133a does not alter TGF-β1-induced MMP-2 or MMP-9 gene expression or enzyme activity, thereby creating microenvironment conditions conducive to promoting the degradation of extracellular matrix.
MiRNAs selectively cleavage or bind to mRNAs to affect their stability or translational activity24,34. We used another bioinformatic tool (www.targetscan.org) to identify possible targets of miR-133a. KEGG analysis of the Targetscan similarly predicted a down-regulation of ECM-related profibrotic genes such as CTGF, collagens, fibronectin, and suggested that the TGF-β signaling pathway is a prominent target. In line with our analysis, Col1a1 was recently identified as a direct target of miR-133a in a different context35. Thus, both predicted direct targets CTGF and Col1a1 or un-predicted targets Col4a1 and α-SMA are down-regulated after miR-133a overexpression, strongly suggesting an anti-fibrotic role of miR-133a in pulmonary fibrosis.
Furthermore, miR-133a treatment of human pulmonary myofibroblasts also upregulates oxidative phosphorylation and the citric acid cycle, which are consistent with miR-133a inducing the formation of a more quiescent fibroblast phenotype (i.e., fibrocyte). Thus, miR-133a treated myofibroblasts have a slenderer spindle shape without α-SMA expression, show decreases in focal adhesion markers and extracellular matrix protein synthesis, and have likely switched to efficient aerobic Krebs cycle metabolism to generate energy rather than utilizing anaerobic glycolysis for cell growth and proliferation. Our observations suggesting miR-133a-induced alterations in cellular metabolism are supported by the findings of impaired exercise tolerance, mitochondrial biogenesis, and skeletal muscle fiber structure and maintenance in miR-133a-deficient mice36,37.
Currently, median survival time for IPF patients is only 2–3 years from diagnosis and limited effective therapies for IPF exist. Thus, there is an unmet need for novel anti-fibrotic therapies. MiRNA-based therapeutic approaches may represent a promising alternative to current approaches in treatment of IPF38,39. The combined data obtained from cultured human pulmonary fibroblasts pre-exposed to TGF-β1 showed that they reverted to a smaller resting phenotype with diminished synthesis of contractile and extracellular matrix proteins following miR-133a treatment. These in vitro findings predicted that selective expression of miR-133a in pulmonary fibroblasts may limit or perhaps even reverse pulmonary fibrosis in vivo. Therefore, we generated a novel fibroblast-targeted miR-133a expression vector and tested our prediction using a mouse model of bleomycin-induced pulmonary fibrosis. Five days after administering bleomycin to mice to induce experimental pulmonary fibrosis, this vector was administered intravenously. Three miR-133a vector treatments caused an easily discernible and significant decrease in bleomycin-induced lung fibrosis and preservation of normal lung structure 10 days later. These data confirm that selective upregulation of miR-133a in pulmonary fibroblasts in vivo exerts therapeutically beneficial anti-fibrotic effects. Although additional work is required to optimize this miRNA-based strategy to inhibit or even reverse pulmonary fibrosis, modulating the expression of miR-133a in fibroblasts may provide a new therapeutic target for pulmonary fibrosis treatment. Further investigation of whether miR-133a deficiency (knockout) exacerbates bleomycin-induced pulmonary fibrosis in mice or in vivo gain of miR-133a (knock-in) protects mice from bleomycin-induced pulmonary fibrosis will provide direct evidence and increase the validity of miR-133a as therapeutics against pulmonary fibrosis.
In conclusion, our study shows that an increase in intracellular miR-133a exerts anti-fibrotic effects on pulmonary fibroblasts. This molecular mediator alters intracellular protein expression in fibroblasts that had been driven by TGF-β1 into the contractile myofibroblast phenotype. Multiple myofibroblast proteins and pathways are targeted during this process with levels of some key proteins being decreased while others are increased. Proteins directly decreased by miR-133a include TGF-β1 receptor, CTGF and collagen that are directly involved in the ability of these cells to transform, proliferate and secrete extracellular matrix in response to TGF-β1 (Fig. 7g). Proteins increased in TGF-β1-stimulated pulmonary fibroblasts by miR-133a include those involved in oxidative phosphorylation. Unchanged by miR-133a upregulation is TGF-β1-stimulated expression of matrix metalloproteases that degrade extracellular matrix. Altogether, this creates an efficiently metabolizing quiescent fibroblast phenotype with reduced extracellular matrix synthesis which should favor removal of extracellular matrix. Anticipated beneficial effects of miR-133a upregulation in pulmonary fibroblasts were found in the bleomycin-induced mouse model of pulmonary fibrosis. These data are an exciting breakthrough given the current dismal prognosis associated with IPF in humans. It should be noted that any therapeutic plan for using miR-133a to treat IPF must include strategies to directly target the fibroblasts due to undesirable off-target effects such as epithelial-to-mesenchymal transitions. Thus, identifying additional fibroblast-selective mechanisms that might increase intracellular miR-133a expression in these cells and potential beneficial/synergistic interactions with other miRNAs that have been associated with IPF should be explored. We are currently collaborating with a medicinal chemist to select the most potent/efficacious compounds that are specific for miR-133a upregulation and action pathways in pulmonary fibroblasts. Of particular interest in pulmonary fibrosis is the fact that these therapies can be selectively delivered to the lung by inhalation. Our long-term goal is to determine if targeting fibroblast miR-133a expression via local drug application provides an effective strategy for prevention and/or treatment of IPF. At a minimum, our study provides legitimate hope that effective therapies for fibrosis-associated morbidity and mortality such as that due to IPF are on the horizon.
Materials and methods
Cell culture, reagents, and antibodies
HEK293 cells and mouse fibroblast NIH3T3 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human primary lung fibroblast cells (HFL) were established by Dr. Reynold Panettieri’s laboratory from patients with brain-related disease but no history of pulmonary fibrosisQuantitative proteomics and bioinformatics analysis Cells were lysed by sonication in 8 M urea/0.1 M Tris-HCl (pH 8.0)/1 × Protease Inhibitor Cocktail (Sigma). Cell lysates were reduced with 10 mM DTT for 2 h at room temperature followed by alkylation with 20 mM iodoacetamide for 30 min in the dark. Samples were trypsinized in 50 mM triethylammonium bicarbonate (TEAB) at 37 °C overnight, then desalted and eluted with 60% acetonitrile. Peptides were lyophilized and re-dissolved with 100 mM TEAB buffer. 100 μg of protein of each sample was labeled with TMT six-plex® (Thermo Fisher Scientific) according to the manufacturer’s instructions. Samples were fractionated using an L-3000 HPLC System (Rigol, Bei**g, China) and all nanoLC-MS/MS experiments were performed on a Q Exactive equipped with an Easy n-LC 1000 HPLC system (Thermo Fisher Scientific) as previously described48. The raw data from Q Exactive were analyzed with Proteome Discovery version 2.2.0.388 using Sequest HT search engine for protein identification and Percolator for FDR (false discovery rate) analysis. The Uniprot Human protein database was used and FDR < 0.05 was set for protein identification. Normalization to the protein median of each sample was used to correct for experimental bias and the normalization mode was selected as total peptide amount. Among the 5394 total proteins detected, differentially regulated proteins (P < 0.05) were identified by multiple ANOVA analysis in conjunction with Dunnet’s analysis using R (3.2) and their associations with signaling pathways in HFL cells were explored using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database resource (www.kegg.jp) and the R package clusterProfiler that automates the process of biological-term classification and the enrichment analysis of gene clusters49. Briefly, genes in each pathway for Homo sapiens were extracted from the KEGG database. Fish’s extract test was further applied to identify enriched pathways (unadjusted P < 0.05). Differentially expressed proteins were further validated by immunoblotting or RT-PCR. Female C57BL/6JCnc mice (6–8 weeks of age) were obtained from Vital River Experimental Animal Center (Bei**g, China) and maintained in a specific pathogen-free environment. All experiments were performed according to the guidelines for experimental animals and approved by the Institutional Animal Care and Use Committee of the Institute of Biophysics, Chinese Academy of Sciences. The mice were intratracheally administered with either bleomycin (50 mg/kg per mouse in 50 μL of saline; Zhejiang Hisun Pharmaceutical Co., Ltd., Taizhou, China) or vehicle (control) on Day 022,40. To overexpress miR-133a specifically in mouse lung fibroblasts, 25 μg of pAAV-FTS1-GFP or pAAV-FTS1-GFP-miR-133a complexed with EntransterTM-in vivo (Engreen Biosystem Co, Ltd., Bei**g, China) was injected via the tail vein on Day 5 and every 4 days thereafter. The mice (n = 4) were killed on Day 15. Lungs were collected and inflated with 4% paraformaldehyde for 2 days and embedded in paraffin before sectioning into 5-μm-thick slices. Sections of paraformaldehyde-fixed mouse lungs were analyzed by hematoxylin and eosin (H&E) or Masson’s trichrome staining to assess fibrotic changes in the lungs as described40. Photos of at least 15 fields from multiple sections of each mouse lung were taken at ×200 magnification and scored separately by the modified Ashcroft method (score range 0–8)40,50,51. The pulmonary fibrosis histopathology score of each mouse is expressed as the mean score of at least 15 photos. All values are expressed as the mean ± SEM of at least three independent experiments. Data were analyzed using the GraphPad Prism software (GraphPad Software, Inc, San Diego, CA, USA). The Student’s unpaired t test (two-tailed) was used for comparison between two groups. *P < 0.05 was considered statistically significant; ns, not significant.Bleomycin-induced mouse pulmonary fibrosis and histologic analysis
Statistical analysis
References
Wynn, T. A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208, 1339–1350 (2011).
Todd, N. W., Luzina, I. G. & Atamas, S. P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5, 11–34 (2012).
Samarakoon, R., Overstreet, J. M. & Higgins, P. J. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 25, 264–268 (2013).
Border, W. A. & Noble, N. A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292 (1994).
Massague, J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 (2012).
Fernandez, I. E. & Eickelberg, O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 9, 111–116 (2012).
Zhang, Y. E. Non-Smad pathways in TGF-beta signaling. Cell Res. 19, 128–139 (2009).
Morikawa, M., Derynck, R. & Miyazono, K. TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 8, a021873 (2016).
He, L. & Hannon, G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 (2004).
Rajasekaran, S., Rajaguru, P. & Gandhi, P. S. S. MicroRNAs as potential targets for progressive pulmonary fibrosis. Front. Pharmacol. 6, 254–268 (2015).
Guo, F., Carter, D. E., Leask, A. & Heldin, C. H. MiR-218 regulates focal adhesion kinase–dependent TGFβ signaling in fibroblasts. Mol. Biol. Cell 25, 1151–1158 (2014).
Cushing, L. et al. MiR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Resp. Cell Mol. Biol. 45, 287–294 (2011).
Miao, C., **ong, Y., Zhang, G. & Chang, J. MicroRNAs in idiopathic pulmonary fibrosis, new research progress and their pathophysiological implication. Exp. Lung Res. 44, 178–190 (2018).
Yang, S. et al. MiR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 27, 2382–2391 (2013).
Liu, G. et al. MiR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 (2010).
Ji, X. et al. The anti-fibrotic effects and mechanisms of microRNA-486-5p in pulmonary fibrosis. Sci. Rep. 5, 14131–14143 (2015).
Cutroneo, K. R., White, S. L., Phan, S. H. & Ehrlich, H. P. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta 1 induced collagen gene expression. J. Cell. Physiol. 211, 585–589 (2007).
Lali, F. V., Hunt, A. E., Turner, S. J. & Foxwell, B. M. J. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem. 275, 7395–7402 (2000).
Hidalgo, M. A. et al. FMLP-induced IL-8 release is dependent on NADPH oxidase in human neutrophils. J. Immunol. Res. 2015, 120348–120363 (2015).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Chen, J. F. et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38, 228–233 (2006).
Walters, D. M. & Kleeberger, S. R. Mouse models of bleomycin-induced pulmonary fibrosis. Curr. Protoc. Pharmacol. 40, 46.1–5.46.17 (2008).
Upagupta, C., Shimbori, C., Alsilmi, R. & Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. 27, 180033 (2018). pii.
Carthew, R. W. & Sontheimer, E. J. Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009).
Thum, T. & Condorelli, G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ. Res. 116, 751–762 (2015).
Zhang, J. et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom. Proteom. Bioinform. 13, 17–24 (2015).
Huang, Y., He, Y. & Li, J. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr. Pharm. Des. 21, 2236–2242 (2015).
Nohata, N., Hanazawa, T., Enokida, H. & Seki, N. MicroRNA-1/133a and microRNA-206/133b clusters: dysregulation and functional roles in human cancers. Oncotarget 3, 9–21 (2012).
Matkovich, S. J. et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ. Res. 106, 166–175 (2010).
Liu, N. et al. MicroRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 22, 3242–3254 (2008).
Chiba, Y., Tanabe, M., Goto, K., Sakai, H. & Misawa, M. Down-regulation of miR-133a contributes to up-regulation of RhoA in bronchial smooth muscle cells. Am. J. Respir. Crit. Care. Med. 180, 713–719 (2009).
Chiba, Y. et al. Interleukin-17A directly acts on bronchial smooth muscle cells and augments the contractility. Pharmacol. Rep. 69, 377–385 (2017).
Chen, L. et al. Upregulated miR-133a orchestrates epithelial-mesenchymal transition of airway epithelial cells. Sci. Rep. 8, 15543–15553 (2018).
Valencia-Sanchez, M. A., Liu, J. D., Hannon, G. J. & Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20, 515–524 (2006).
Castoldi, G. et al. MiR-133a regulates collagen 1A1: potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. J. Cell Physiol. 227, 850–856 (2012).
Liu, N. et al. Mice lacking microRNA 133a develop dynamin 2-dependent centronuclear myopathy. J. Clin. Invest. 121, 3258–3268 (2011).
Nie, Y. et al. Impaired exercise tolerance, mitochondrial biogenesis, and muscle fiber maintenance in miR-133a-deficient mice. FASEB J. 30, 3745–3758 (2016).
Broderick, J. A. & Zamore, P. D. MicroRNA therapeutics. Gene Ther. 18, 1104–1110 (2011).
Chakraborty, C., Sharma, A. R., Sharma., G., Doss, C. G. P. & Lee, S. S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol. Ther. Nucleic Acids 8, 132–143 (2017).
**e, Y. et al. Upregulation of RGS2: a new mechanism for pirfenidone amelioration of pulmonary fibrosis. Respir. Res. 17, 103–116 (2016).
Chen, C. et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 33, 179–187 (2005).
Agarwal, V., Bell, G. W., Nam, J. W. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 4, e05005 (2015).
**e, Y. et al. Regulator of G protein signaling 2 is a key modulator of airway hyperresponsiveness. J. Allergy Clin. Immunol. 130, 968–976 (2012).
Wong, C. Y. et al. Phorbol myristate acetate suppresses breast cancer cell growth via down-regulation of P-Rex1 expression. Protein Cell 7, 445–449 (2016).
Okada, H. et al. Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. Am. J. Physiol. 275, F306–F314 (1998).
Strutz, F. et al. Identification and characterization of a fibroblast marker - Fsp1. J. Cell Biol. 130, 393–405 (1995).
Toth, M., Sohail, A. & Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 878, 121–135 (2012).
Ding, C. et al. A fast workflow for identification and quantification of proteomes. Mol. Cell. Proteomics 12, 2370–2380 (2013).
Yu, G. C., Wang, L. G., Han, Y. Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Hubner, R. H. et al. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 44, 507–511 (2008). 514–507.
Ashcroft, T., Simpson, J. M. & Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 41, 467–470 (1988).
Acknowledgements
This work was supported in part by the National Laboratory of Biomacromolecules and the Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences (CAS); the CAS/SAFEA International Partnership Program for Creative Research Teams and the National Natural Science Foundation of China (31671175) (To T.W.); the National Institutes of Health (R01HL116849) and Nebraska LB595 Research Program, USA (To Y.T.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. Calin
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, P., **e, Y., Abel, P.W. et al. Transforming growth factor (TGF)-β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis 10, 670 (2019). https://doi.org/10.1038/s41419-019-1873-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1873-x
- Springer Nature Limited
This article is cited by
-
Inhibitor of PD-1/PD-L1: a new approach may be beneficial for the treatment of idiopathic pulmonary fibrosis
Journal of Translational Medicine (2024)
-
Aging and homeostasis of the hypodermis in the age-related deterioration of skin function
Cell Death & Disease (2024)
-
TMT proteomics analysis reveals the mechanism of bleomycin-induced pulmonary fibrosis and effects of Ginseng honeysuckle superfine powdered tea
Chinese Medicine (2023)
-
Exosomal miR-218 derived from mesenchymal stem cells inhibits endothelial-to-mesenchymal transition by epigenetically modulating of BMP2 in pulmonary fibrosis
Cell Biology and Toxicology (2023)
-
CircUBE2Q2 promotes differentiation of cattle muscle stem cells and is a potential regulatory molecule of skeletal muscle development
BMC Genomics (2022)