Abstract
Epstein–Barr virus (EBV) was the first oncogenic virus identified in humans. It is primarily associated with multiple lymphoid and epithelial cancers, including nasopharyngeal carcinoma (NPC). However, its association with ferroptosis and its role in cancer therapy resistance have not been fully elucidated. Here, we show that EBV infection reduces the sensitivity of NPC cells to ferroptosis by activating the p62-Keap1-NRF2 signaling pathway in conjunction with upregulation of SLC7A11 and GPX4 expression. Knockdown of endogenous GPX4 or blockade of GPX4 using a specific inhibitor enhanced the chemosensitivity of EBV-infected NPC cells. Functional studies revealed that GPX4 knockdown suppresses the proliferation and colony formation of NPC cells. Mechanistically, GPX4 interacts with the TAK1-TAB1/TAB3 complex, regulates TAK1 kinase activity, and further activates downstream MAPK-JNK and NFκB pathways. High GPX4 expression is correlated with poor clinical outcomes in patients with NPC and other cancer types. Taken together, our findings suggest that EBV infection has important effects on redox homeostasis, revealing a previously unappreciated role for GPX4 in tumor progression. This novel mechanism provides a potential new target for the treatment of EBV-related tumors.
Similar content being viewed by others
Introduction
Epstein–Barr virus (EBV) is a large double-stranded DNA virus that belongs to the gammaherpesvirinae subfamily [1]. As the first identified human oncogenic virus, EBV contributes to approximately 1.5% of all cases of cancer worldwide, including lymphoid and epithelial cancers [2,3,4]. The malignancy that is most closely associated with EBV infection is undifferentiated nasopharyngeal carcinoma (NPC), which occurs in the epithelial lining of the nasopharynx [5,6,7]. Chemoradiotherapy is the fundamental treatment strategy for NPC. However, the treatment of NPC still faces great challenges due to chemoresistance [8].
EBV is consistently detected in NPC patients from both endemic and non-endemic area. Delineating the cellular processes targeted by EBV is essential to understand its role in tumor initiation and progression and may contribute to the discovery of new therapeutic targets. Increasing evidence has emerged to reveal the functions of viral proteins (e.g., EBVN1 and LMP1) and small RNAs that may contribute to EBV-associated cancers [9, 10]. However, the role of EBV infection in the mechanism of chemoresistance has not been fully elucidated.
Ferroptosis, a nonapoptotic form of cell death, is featured by the excessive iron-dependent accumulation of lipid reactive oxygen species (ROS) [11,12,13]. The distinctive characteristics of cells undergoing ferroptosis include a series of morphological abnormalities of mitochondria, such as organelle shrinkage, condensed organelle membrane, and lessened cristae. Ferroptosis is precisely controlled via a regulatory network involving the inhibitory role of glutathione peroxidase 4 (GPX4) and cystine transporter SLC7A11 (system Xc-, xCT). GPX4 helps to clear the toxic lipid hydroperoxides (LOOH), which prevents cellular damage from oxidative stress and maintains redox homeostasis [14], while the inhibition of GPX4 promotes lipid ROS-dependent ferroptosis. Cancer cells are known to develop iron addiction, which increases the ROS production as a result of cellular transformation and tumorigenesis [15]. In addition to their increased antioxidant capacity, cancer cells are rendered more susceptible to ferroptosis due to their altered redox environment. Therefore, cancer cells are more dependent on GPX4, especially following epithelial-to-mesenchymal transition (EMT) [16, 17]. Directly targeting GPX4 may serve as an efficient strategy to induce ferroptosis in cancer cells in vivo and provide a new approach for ROS manipulation-based cancer therapy.
To the best of our knowledge, the effect of EBV infection on the ferroptosis sensitivity of host cells has not been studied to date. Here, we show that EBV infection activates the p62-Keap1-NRF2 signaling axis, leading to upregulation of GPX4 and SLC7A11, and effectively reduces the ferroptosis sensitivity of NPC cells. Inhibition of GPX4 leads to enhanced chemosensitivity in EBV-infected NPC cells. Additionally, GPX4 knockdown significantly suppresses tumor cell proliferation in vitro and in vivo. We further demonstrated that GPX4 interacts with the TAK1-TAB1/TAB3 complex, regulates TAK1 kinase activity, and activates the downstream MAPK-JNK and NFκB pathways. Altogether, our findings uncovered a novel mechanism by which chemoresistance induced by EBV infection facilitates the evasion of ferroptosis, identifying GPX4 is a potential therapeutic target in NPC.
Results
EBV infection reduces ferroptosis in NPC cells
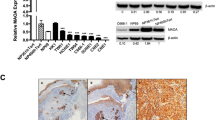
To explore the effect of EBV infection on the ferroptosis sensitivity of nasopharyngeal epithelial cells, we developed EBV-infected NPC cell lines as previously described [Full size image
TAK1 is a member of the mitogen-activated protein (MAP) kinase kinase kinase (MAP3K) family [30], and it functions as an intracellular hub that modulates not only MAP kinase, but also the nuclear factor-κB (NF-κB), thereby regulating multiple vital biological progresses [ To analyze GPX4 expression and GPX4-related survival, we retrospectively collected 181 paraffin-embedded NPC specimens from Sun Yat-sen University Cancer Centre between November 2010 and November 2011. All patients were diagnosed with nonmetastatic NPC, and none of the patients had received radiotherapy or chemotherapy before biopsy. The 7th edition of the AJCC Cancer Staging Manual was used to reclassify TNM stages. The median follow-up period was 62 months (range, 4–84 months). The pathologic type was WHO III in all cases. All patients received radiotherapy, and patients with stage III–IV NPC received inducing chemotherapy plus concurrent platinum-based chemotherapy or chemotherapy alone. The detailed clinicopathological characteristics are shown in Supplementary Table S1. This study was approved by the Institutional Ethical Review Board of the Sun Yat-sen University Cancer Centre (No. GZR2020-090 for human cancer specimens and NO. L102012020000N for in vivo mouse experiments). Written informed consent was obtained from all patients. Akata-EBV-GFP is an Akata Burkitt lymphoma cell line carrying the Akata bacterial artificial chromosome (BAC) with a GFP tag and was cultured in RPMI 1640 medium with 10% foetal bovine serum (FBS) (HyClone). NPC cell lines (HK1, HK1-EBV, CNE2, CNE2-EBV) were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 5% foetal bovine serum (FBS) in a humidified atmosphere comprising 5% CO2 at 37 °C. The EBV-positive Akata cell line and all NPC cell lines, which had been authenticated, were kindly provided by Dr. Mu-sheng Zeng (Sun Yat-sen University Cancer Centre). 293FT cells obtained from ATCC were grown in DMEM (Invitrogen) with 10% FBS. All cells were authenticated using short-tandem repeat profiling, tested for mycoplasma contamination, and cultured for less than 2 months. The following antibodies were used in this study: anti-4-hydroxynonenal (4-HNE), Abcam (ab46545), for immunoblotting and IHC; anti-p62, Abcam (ab109012), for immunoblotting; anti-NRF2, Abcam (ab62352), for immunoblotting and Cell Signaling Technology (#12721), for immunofluorescence staining; anti-Keap1, Abcam (ab227828), for immunoblotting; anti-SLC7A11, Abcam (ab175186), for immunoblotting; anti-GPX4, Abcam (ab125066), for immunoblotting and IHC; anti-GAPDH, Cell Signaling Technology, #5174, for immunoblotting; anti-β-actin, Abcam (ab8226), for immunoblotting; anti-TAK1, Abcam (ab109526), for immunoblotting; anti-p-TAK1(T187), Cell Signaling Technology, #4536, for immunoblotting; anti-TAB1, Abcam (ab76412), for immunoblotting and immunofluorescence staining; anti-TAB3, Abcam (ab124723), for immunoblotting and immunofluorescence staining; anti-Flag, Sigma (F1804), for immunoblotting and immunofluorescence staining; anti-IKKα, Cell Signaling Technology, #11930, for immunoblotting; anti-IKKβ, Cell Signaling Technology, #8943, for immunoblotting; anti-p-IKKα/β(Ser176/180), Cell Signaling Technology, #2697, for immunoblotting; anti-p-NFκB (Ser536), Cell Signaling Technology, #3033, for immunoblotting; anti-NFκB, Cell Signaling Technology, #8242, for immunoblotting; anti-p-IκBα (Ser32), Cell Signaling Technology, #2859, for immunoblotting; anti-IκBα (Ser32), Cell Signaling Technology, #4814, for immunoblotting; anti-p-JNK(Thr183/Tyr185), Cell Signaling Technology, #4668, for immunoblotting; anti-JNK, Cell Signaling Technology, #9252, for immunoblotting; anti-p-p38(Thr180/Tyr182), Cell Signaling Technology, #4511, for immunoblotting; and anti-p38, Cell Signaling Technology, #8690, for immunoblotting. BODIPY 581/591 C11 (Lipid Peroxidation Sensor), Invitrogen (D3861); erastin, Sigma (E7781); Ferrostatin-1, Sigma (SML0583); 1 S,3R-RSL3, Sigma (SML2234); and SYTOX Kit, Invitrogen (S34862) were also utilized. EBV with integrated EGFP (EBV-EGFP) was prepared in Akata cells as previously described [43]. The Akata-EBV-GFP cell line was used to produce EBV in accordance with the following procedure: 0.8% (v/v) goat anti-human IgG was used to treat the cells for 6 h to induce EBV into a lytic cycle, and then the cells were cultured in fresh medium for 3 days. Next, supernatants of the cells were centrifuged at 800×g for 30 min to remove cellular debris. Then, the supernatants were centrifuged at 50,000×g for 2 h to obtain EBV pellets. EBV pellets were resuspended in RPMI-1640 and used to infect NPC cells. EBV-infected CNE2 and HK1 cells were sorted by flow cytometry and cultured in RPMI-1640 medium using a various concentration (300–700 μg mL−1) of G418 (Sigma–Aldrich). EBV infection efficiency was verified by EBER detection using in situ hybridization (ISH) according to the manufacturer’s instructions of the ISH kit for EBER (Zhongshan **qiao Bio. Co.) or confocal laser-scanning microscopy (Olympus FV1000). For EBNA1 knockout cell line construction, the plasmid lentiCRISPR-v2-EBNA1 for EBNA deletion was kindly supplied by Prof. **ang (**ang Tong, Sun Yat-Sen University Cancer Centre) and has been previously described [44]. For lentiviral packaging, the lentiviral vector and packaging plasmids (pMD2.G and psPAX2) were both transfected into HEK293T cells; 48 h later, the virus in the supernatant was collected and used to infect EBV-positive cells. Puromycin (2 μg mL−1) was added to select stable EBNA1-deleted cells. GFP-expressing cells were detected to assess EBV clearance efficiency. Cell death was detected using the SYTOX dead cell stain sampler kit (#S34862, Invitrogen) and the Annexin V-PI apoptosis assay kit (#556547, BD). Briefly, the indicated cells were plated in 12-well plates and treated with different concentrations of cytotoxic compounds for the indicated times. Then, SYTOX stain was added to the cell supernatants, and the cells were incubated at room temperature for 10 min, followed by observation and imaging using a fluorescence microscope. The Annexin V-PI apoptosis assay was quantified using flow cytometry. Lipid ROS production was detected by flow cytometry using C11-BODIPY dye (#D-3861, Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions. Briefly, cells were seeded into 12-well plates and cultured in a 37 °C incubator with 5% CO2. After the cells were treated with different cytotoxic reagents for the indicated times, C11-BODIPY was added to the cell supernatants, and the cells were cultured for more than 30 min before ROS detection. ROS can oxidize the polyunsaturated butadienyl portion of C11-BODIPY, and then the fluorescence emission peak of the dye shifts from ~590 nm to ~510 nm. The pEZ-Lv105-puro-vector and GPX4 plasmids were purchased from GeneCopoeia, Inc., USA. The sequence of the human GPX4 gene was synthesized and cloned into the lentiviral plasmid pEZ-Lv105-puro-vector. The primers used for amplification were as follows: GPX4-Forward 5′-ATGAGCCTCGGCCGCCTTTG-3′; GPX4-Reverse 5′-CCCACAAGGTAGCCAGGGGT-3′. GPX4 shRNA sequences were as follows: shGPX4 1#: GCTACAACGTCAAATTCGA shGPX4 2#: GAGGCAAGACCGAAGTAAA To generate stably transfected cell lines, 293FT cells were cotransfected with the lentivirus packaging vector and shRNA knockdown plasmids. The lentiviral particles were subsequently harvested and used to infect NPC cells 48 h later. Stable clones were then selected using 1 mg/mL puromycin (Sigma–Aldrich), and real-time RT–PCR or western blot assays were used to validate the infection efficiency. Effective siRNA oligonucleotides targeting TAK1 and NRF2 were obtained from RiboBio with the following sequences: siTAK1-#1: GGAGTGGCTTATCTTCACA; siTAK1-#2: GGCTTATCTTACACTGGAT; siNRF2-1#: GAGAAAGAATTGCCTGTAA; siNRF2-2#: GCTACGTGATGAAGATGGA; siNRF2-3#: GCCCTCACCTGCTACTTTA. The negative control (siCtrl) was nonhomologous to any human genome sequence and purchased from RiboBio Co., Ltd. Predetermined cells were grown in 6-well plates for 12 h and then transfected with 20 nM siRNA mixed with 5 μL of Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. Thirty-six to 48 h after transfection, the cells were harvested for further analysis. Total RNA was extracted using TRIzol (Life Technology), reverse transcribed into cDNA using the GoScriptTM Reverse Transcription System (Promega) and analyzed by real-time qPCR on a BIO-RAD Real-time PCR machine using iTaq™ Universal SYBR® Green Super mix (Bio–Rad). The results were normalized to β-actin, and relative values were calculated using the 2[-(CT gene -CT reference)] method. The gene-specific primers used for qPCR are listed in Supplementary Table 3. Total protein was obtained using RIPA buffer (Beyotime Biotechnology) containing EDTA-free Protease Inhibitor Cocktail (Roche). Protein extracts were separated on 10–12% acrylamide SDS–PAGE and transferred to polyvinylidene fluoride membranes (Merck Millipore). The membranes were blocked in 5% non-fat milk and incubated with primary antibodies overnight at 4 °C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (anti-mouse or anti-rabbit; 1:3,000; Thermo) at room temperature for 45 min. Finally, the target protein bands were detected using an enhanced chemiluminescence system (Thermo Fisher Scientific). For the immunoprecipitation (IP) assay, HK1 cells transfected with the empty vector or Flag-GPX4 expression plasmid were lysed in IP lysis buffer. ANTI-FLAG® M2 Affinity Gel (Sigma, A2220-10 ML) was incubated with the lysates overnight at 4 °C and then washed and collected according to the manufacturer’s protocol. Mass spectrometry was performed by Huijun Biotechnology. For the co-IP assay, western blotting was performed to determine protein levels. Cells were rinsed in PBS and then lysed in IP lysis buffer. The protein extracts were subsequently incubated with the ANTI-FLAG® M2 Affinity Gel. The precipitated proteins were separated and detected by western blotting using the indicated antibodies. Finally, the blots were visualized using a chemiluminescence system. For the GST pull-down assay, recombinant human GPX4 protein expressed in Escherichia coli was purchased from Abcam company (ab82660), and GST-TAK1 (full length 1-606 aa or N-terminal 1-305 aa) was expressed in Rossita. GST-TAK1 or GST-vector and GPX4 were incubated in lysis buffer overnight at 4 °C. GST-agarose was added to lysis buffer, incubated for 4 h at 4 °C, washed four times with lysis buffer and analyzed by immunoblotting. For immunofluorescence staining, cells were grown on coverslips (Thermo Fisher Scientific). After 24 h, the cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and incubated with primary anti-NRF2 (1:200; Cell Signaling Technology (#12721)), anti-Flag (1:200; Sigma, F1804), anti-MAP3K7 (1:1000; Abcam, ab109502), anti-TAB1 (1: 400; Abcam, ab76412), and anti-TAB3 (1: 50; Abcam, ab124723) antibodies overnight at 4 °C. The coverslips were then incubated with Alexa Fluor 488- or 594-conjugated goat IgG secondary antibodies (1:1,000; Life Technologies; A-11008 or A-11001) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were captured using a confocal laser-scanning microscope (Olympus FV1000). Expression levels of GPX4 in patient tissues were detected by IHC in paraffin-embedded sections. Briefly, the sections were deparaffinized in xylene, rehydrated in a graded alcohol series, incubated in 3% hydrogen peroxide to block endogenous peroxidase activity, heated in antigen retrieval solution, subjected to FBS to block nonspecific binding, and incubated with GPX4 antibody (Abcam, ab125066, 1:100) at 4 °C overnight. Two experienced pathologists validated the scores of all sections. Cell death was analyzed using propidium iodide (Invitrogen, Waltham, MA, USA) or SYTOX Green (Invitrogen) staining followed by microscopy or flow cytometry. Viability was measured using the CCK-8 assay. To analyze lipid peroxidation, cells were stained with 5 μM BODIPY-C11 (Invitrogen) for 30 min at 37 °C followed by flow cytometric analysis. Lipid ROS-positive cells were defined as cells with fluorescence greater than 99% of the cells in the unstained sample. Cell growth was measured using a CCK-8 kit (Sigma, St Louis, MO, USA) according to the instructions. Briefly, approximately 1500 cells were plated into 96-well plates and cultured in the indicated medium. Cell proliferation measured at 450 nm (A450) was examined every day for five days according to the manufacturer’s protocol. All experiments were performed three times. The EdU incorporation assay was performed as previously described [45]. Briefly, 5 × 104 cells were seeded onto coverslips in 24-well plates. After reaching 80% confluence, EdU (20 μM) was added to the supernatants of cells and cultured for 1.5 h, followed by the click reaction according to the manufacturer’s instructions. Then, the cells were assessed under a confocal microscope to calculate the positive rate of EdU incorporation. The cell cycle was evaluated using propidium iodide (PI) staining and quantified using a Gallios flow cytometer. Treated cells (400 cells/well) were plated in triplicate into 6-well plates and cultured for 10 days. The colonies were fixed in methanol for 15 min and stained with crystal violet for another 15 min. Finally, the colony number was quantified. All experiments were performed three times. All data are presented as the means ± SD from at least three independent experiments. Two-tailed unpaired Student’s t test was used for statistical analysis involving two group comparisons (ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Data with a non-Gaussian distribution were compared using a two-tailed Mann–Whitney test, which was performed for the growth curves of the indicated xenografts. Differences with p < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). The Kaplan–Meier method and log-rank test were used to construct survival curves and compare the differences, respectively. Independent prognostic factors were determined by multivariate analysis using a Cox proportional hazards regression model. A chi-squared (χ2) test was performed to determine the correlation between EBV copy number and GPX4 expression. Statistical analyses were performed using SPSS 22.0 software (IBM).Materials and methods
Clinical specimens
Cell culture
Antibodies and reagents
Virus preparation and infection
Constructs and CRISPR/Cas9-mediated EBNA1 deletion
Cell death analysis
Lipid ROS assay
Oligonucleotides, plasmids, and stable cell lines
siRNA transfection and qRT–PCR
Western blot assay
Coimmunoprecipitation (co-IP) and mass spectrometry
GST pull-down assay
Immunofluorescence staining
IHC staining
Measurement of cell death, cell viability and lipid peroxidation
CCK-8 assay
Cell cycle analysis and EdU incorporation analysis
Colony formation assay
Statistical analysis
Data availability
Gene-expression profiling interactive analysis in different cancer types and corresponding normal tissues were deposited at GEPIA based on TCGA and GTEx data (http://www.ncbi.nlm.nih.gov/geo/). The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the approval RDD number as RDDB2021319938. Scripts and additional data related to this work will be available upon request to the corresponding author.
References
Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68.
Tsao S-W, Tsang CM, To K-F, Lo K-W. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015;235:323–33.
Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15:266–82.
Tsang CM, Deng W, Yip YL, Zeng MS, Lo KW, Tsao SW. Epstein-Barr virus infection and persistence in nasopharyngeal epithelial cells. Chin J Cancer. 2014;33:549–55.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin. 2015;65:87–108.
Li SB, Liu YY, Yuan L, Ji MF, Zhang A, Li HY, et al. Autocrine INSL5 promotes tumor progression and glycolysis via activation of STAT5 signaling. EMBO Mol Med. 2020;12:e12050.
Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol Cancer. 2007;6:1.
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol: Off J Am Soc Clin Oncol. 1998;16:1310–7.
Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6:e1000940.
Ye Y, Zhou Y, Zhang L, Chen Y, Lyu X, Cai L, et al. EBV-miR-BART1 is involved in regulating metabolism-associated genes in nasopharyngeal carcinoma. Biochemical Biophysical Res Commun. 2013;436:19–24.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060–72.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017;171:273–85.
Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156:317–31.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009;458:780–3.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91.
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617.
**ong D, Du Y, Wang HB, Zhao B, Zhang H, Li Y, et al. Nonmuscle myosin heavy chain IIA mediates Epstein-Barr virus infection of nasopharyngeal epithelial cells. Proc Natl Acad Sci USA. 2015;112:11036–41.
Hu J, Li Y, Li H, Shi F, **e L, Zhao L, et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated high oxidative stress suppresses EBV lytic reactivation and sensitizes tumors to radiation therapy. Theranostics 2020;10:11921–37.
Lee CH, Fang CY, Sheu JJ, Chang Y, Takada K, Chen JY. Amplicons on chromosome 3 contain oncogenes induced by recurrent exposure to 12-O-tetradecanoylphorbol-13-acetate and sodium n-butyrate and Epstein-Barr virus reactivation in a nasopharyngeal carcinoma cell line. Cancer Genet Cytogenet. 2008;185:1–10.
Tang X, Wu J, Ding CK, Lu M, Keenan MM, Lin CC, et al. Cystine deprivation triggers programmed necrosis in VHL-deficient renal cell carcinomas. Cancer Res. 2016;76:1892–903.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25.
Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21:363–81.
Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, et al. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84.
Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–203.
Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E, et al. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab. 2021;33:174–89. e7
Wu WL, Papagiannakopoulos T. The center cannot hold: NRF2 battles ferroptosis in the 3rd dimension. Mol Cell. 2020;80:760–1.
De Leo A, Calderon A, Lieberman PM. Control of viral latency by episome maintenance proteins. Trends Microbiol. 2020;28:150–62.
van Diemen FR, Kruse EM, Hooykaas MJ, Bruggeling CE, Schurch AC, van Ham PM, et al. CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog. 2016;12:e1005701.
Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR 3rd, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–9.
Ajibade AA, Wang Q, Cui J, Zou J, **a X, Wang M, et al. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity 2012;36:43–54.
Xu YR, Lei CQ. TAK1-TABs complex: a central signalosome in inflammatory responses. Front Immunol. 2020;11:608976.
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–24.
Adelstein D, Gillison ML, Pfister DG, Spencer S, Adkins D, Brizel DM, et al. NCCN guidelines insights: head and neck cancers, Version 2.2017. J Natl Compr Canc Netw. 2017;15:761–70.
Yuen KS, Wang ZM, Wong NM, Zhang ZQ, Cheng TF, Lui WY, et al. Suppression of Epstein-Barr virus DNA load in latently infected nasopharyngeal carcinoma cells by CRISPR/Cas9. Virus Res. 2018;244:296–303.
Kim JH, Kim WS, Yun Y, Park C. Epstein-Barr virus latent membrane protein 1 increases chemo-resistance of cancer cells via cytoplasmic sequestration of Pim-1. Cell Signal. 2010;22:1858–63.
Yang GD, Huang TJ, Peng LX, Yang CF, Liu RY, Huang HB, et al. Epstein-Barr Virus_Encoded LMP1 upregulates microRNA-21 to promote the resistance of nasopharyngeal carcinoma cells to cisplatin-induced Apoptosis by suppressing PDCD4 and Fas-L. PLoS One. 2013;8:e78355.
Liu Y, Jiang Q, Liu X, Lin X, Tang Z, Liu C, et al. Cinobufotalin powerfully reversed EBV-miR-BART22-induced cisplatin resistance via stimulating MAP2K4 to antagonize non-muscle myosin heavy chain IIA/glycogen synthase 3beta/beta-catenin signaling pathway. EBioMedicine 2019;48:386–404.
Yun SM, Kim YS, Hur DY. LMP1 and 2A induce the expression of Nrf2 through Akt signaling pathway in Epstein-Barr Virus-transformed B cells. Transl Oncol. 2019;12:775–83.
Nakamura H, Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112:3945–52.
Kim SY, Kim TJ, Lee KY. A novel function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett. 2008;582:1913–8.
Kim YJ, Lee WS, Ip C, Chae HZ, Park EM, Park YM. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006;66:7136–42.
Speck P, Longnecker R. Epstein-Barr virus (EBV) infection visualized by EGFP expression demonstrates dependence on known mediators of EBV entry. Arch Virol. 1999;144:1123–37.
**ang T, Lin YX, Ma W, Zhang HJ, Chen KM, He GP, et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun. 2018;9:5009.
Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105:2415–20.
Acknowledgements
The EBV-positive Akata cell line and EBV-negative and EBV-positive NPC cell lines (HK1 and CNE2) were a kind gift from Prof. Musheng Zeng (Sun Yat-sen University Cancer Centre, Guangzhou, P. R. China). The two gRNAs targeting EBNA1 were a kind gift from TX (Sun Yat-sen University Cancer Centre, Guangzhou, P. R. China).
Funding
This study was funded by grants from the National Key R&D Program of China (2017YFC0908500, 2017YFC1309003), the National Natural Science Foundation of China (81425018, 81672868, 81802775, 82073003, 82002852, 82003267), China Postdoctoral Science Foundation (2019M663252), the Sci-Tech Project Foundation of Guangzhou City (201707020039), the Sun Yat-sen University Clinical Research 5010 Program (2019023), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Natural Science Foundation of Guangdong Province (2017A030312003, 2018A0303131004), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholars (2018B030306001), the Sci-Tech Project Foundation of Guangdong Province (2014A020212103), the Health & Medical Collaborative Innovation Project of Guangzhou City (201400000001, 201803040003), Pearl River S&T Nova Program of Guangzhou (201806010135), the Planned Science and Technology Project of Guangdong Province (2019B020230002), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B10), Natural Science Foundation of Guangdong Province (2017A030312003), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
LT and HM conceived and designed the experiments, provided supervision, and wrote the manuscript. LY, SL, and QC designed and performed most of the experiments, analyzed the data, and created the figures. TX, DL, SL, SG, LL, CD, GJ, XL, and ZY participated in some experiments. LL and HL helped to revise the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
COMPETING INTERESTS
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Ethical Review Board of the Sun Yat-sen University Cancer Centre (No. GZR2020-090 for human cancer specimens and NO. L102012020000N for in vivo mouse experiments). Written informed consent was obtained from all subjects.
Consent for publication
The authors confirm that they obtained written consent from each patient to publish the findings.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by M. Hardwick
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, L., Li, S., Chen, Q. et al. EBV infection-induced GPX4 promotes chemoresistance and tumor progression in nasopharyngeal carcinoma. Cell Death Differ 29, 1513–1527 (2022). https://doi.org/10.1038/s41418-022-00939-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-022-00939-8
- Springer Nature Limited