Abstract
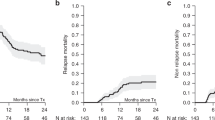
While in vivo T-cell depletion (TCD) is widely used, its benefit in patients with MDS still remains a matter of debate. This study evaluates the impact of TCD on outcomes, and compares ATG and alemtuzumab, in patients with MDS. 1284 patients from the EBMT registry were included in this study with 470 patients in the no-TCD group and 814 in the TCD group (alemtuzumab N = 168; ATG N = 646). At 6 months, aGVHD III-IV cumulative incidences (CI) for no-TCD, ATG or alemtuzumab groups were 13% vs 14% vs 11% (ns), respectively. At 5 years, CI of chronic GVHD were 64% vs 52% vs 51% (p < 0.00017); and CI of relapse was 23% vs 25% vs 39% (p < 0.0001) for no TCD, ATG and alemtuzumab respectively; OS was 47% vs 46% vs 34% (p = 0.009) respectively; and GRFS was 21% vs 28% and 20% (p = 0.045) respectively. In multivariable analysis, ATG improved GRFS, and alemtuzumab decreased OS. Both ATG and alemtuzumab decreased risk of chronic GVHD, but the increased risk of relapse with alemtuzumab is associated with a poor GRFS and suggest to not use alemtuzumab in the setting of allo-SCT for high risk disease.


Similar content being viewed by others
References
Ades L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383:2239–52.
Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer. 2017;17:5–19.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: Contemporary review and how we treat. Am J Hematol. 2016;91:76–89.
de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129:1753–62.
Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–11.
Schetelig J, de Wreede LC, van Gelder M, Koster L, Finke J, Niederwieser D, et al. Late treatment-related mortality versus competing causes of death after allogeneic transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia. Leukemia. 2019;33:686–95.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–2947.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374:43–53.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–11.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–99.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–38.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–11.
Kroger N, Iacobelli S, Franke GN, Platzbecker U, Uddin R, Hubel K, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35:2157–64.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
Juliusson G, Theorin N, Karlsson K, Frodin U, Malm C. Subcutaneous alemtuzumab vs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transpl. 2006;37:503–10.
Kroger N, Shaw B, Iacobelli S, Zabelina T, Peggs K, Shimoni A.Clinical Trial Committee of the British Society of Blood and Marrow Transplantation and the German Cooperative Transplant Group et al. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol. 2005;129:631–43.
Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Kim S-H, et al. Infectious complications associated with alemtuzumab use for allogeneic hematopoietic stem cell transplantation: comparison with anti-thymocyte globulin. Transpl Infect Dis. 2009;11:413–23.
Willemsen L, Jol-van der Zijde CM, Admiraal R, Putter H, Jansen-Hoogendijk AM, Ostaijen-Ten Dam MM, et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transpl. 2015;21:473–82.
Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–70.
Robin M, Raj K, Chevret S, Gauthier J, de Lavallade H, Michonneau D, et al. Alemtuzumab vs anti-thymocyte globulin in patients transplanted from an unrelated donor after a reduced intensity conditioning. Eur J Haematol. 2018;101:466–74.
Cho BS, Kim YJ, Cho SG, Kim SY, Eom KS, Kim HJ, et al. The beneficial effect of chronic graft-versus-host disease on the clinical outcome of transplantation with fludarabine/busulfan-based reduced-intensity conditioning for patients with de novo myelodysplastic syndrome. Int J Hematol. 2007;85:446–55.
Poire X, van Besien K. Alemtuzumab in allogeneic hematopoetic stem cell transplantation. Expert Opin Biol Ther. 2011;11:1099–111.
Bonifazi F, Olivieri J, Sessa M, Dan E, Sinigaglia B, Rizzi S, et al. Low-dose anti-T lymphoglobulin as prophylaxis for graft-versus-host disease in unrelated donor transplantations for acute leukemias and myelodysplastic syndromes. Biol Blood Marrow Transpl. 2018;24:2450–58.
Park SS, Jeon YW, Min GJ, Park S, Yahng SA, Yoon JH, et al. Graft-versus-host disease-free, relapse-free survival after allogeneic stem cell transplantation for myelodysplastic syndrome. Biol Blood Marrow Transpl. 2019;25:63–72.
Author information
Authors and Affiliations
Contributions
Contribution: EF and MR designed the study; SC performed statistical analysis; LK provided data management; JF, GE, FA, DB, AG, GS, LV, HS, MM, JT, PJ, MJPC, YK, MA, AT, PA, TdW, IYA, NK and MR provided patients, reviewed and edited the manuscript. All co-authors reviewed and approved the last version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
NK: research grant from Neovii; MA: Board speaker for Neovii. EF: travel grant from Neovii.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
List of centers contributing to the DQI-EBMT MDS registry:
Rigshospitalet, Copenhagen, Denmark; Hopital St Louis, Paris, France; University Hospital, Essen, Germany; Hannover Medical School, Hannover, Germany; Klinikum Grosshadern, Munich, Germany, HUCH Comprehensive Cancer Center, Helsinky, Finland; Hospital Regional de Malaga, Malaga, Spain; University Hospital Eppendorf, Hamburg, Germany; Centre Hospitalier de Lyon, Lyon, France; Charles University Hospital, Pilsen, Czech Republic; Universitaetsklinikum Dresden, Dresden, Germany; Faculty of Medicine, University of Freiburg, Freiburg, Germany; Medical Park Hospitals, Antalya, Turkey; Central Clinical Hospital, Warsaw, Poland; Florence Nightingale Sisli Hospital, Istanbul, Turkey.
Supplementary information
Rights and permissions
About this article
Cite this article
Forcade, E., Chevret, S., Finke, J. et al. Impact of in vivo T-cell depletion in patients with myelodysplastic syndromes undergoing allogeneic hematopoietic stem cell transplant: a registry study from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 57, 768–774 (2022). https://doi.org/10.1038/s41409-022-01620-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01620-x
- Springer Nature Limited
This article is cited by
-
Impact of post-transplant cyclophosphamide (PTCy)-based prophylaxis in matched sibling donor allogeneic haematopoietic cell transplantation for patients with myelodysplastic syndrome: a retrospective study on behalf of the Chronic Malignancies Working Party of the EBMT
Bone Marrow Transplantation (2024)
-
Sequential vs myeloablative vs reduced intensity conditioning for patients with myelodysplastic syndromes with an excess of blasts at time of allogeneic haematopoietic cell transplantation: a retrospective study by the chronic malignancies working party of the EBMT
Bone Marrow Transplantation (2024)