Abstract
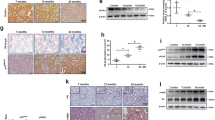
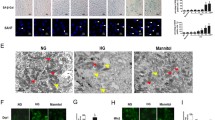
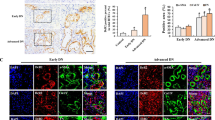
Renal tubular epithelial cell senescence plays a critical role in promoting and accelerating kidney aging and age-related renal fibrosis. Senescent cells not only lose their self-repair ability, but also can transform into senescence-associated secretory phenotype (SASP) to trigger inflammation and fibrogenesis. Recent studies show that mitochondrial dysfunction is critical for renal tubular cell senescence and kidney aging, and calcium overload and abnormal calcium-dependent kinase activities are involved in mitochondrial dysfunction-associated senescence. In this study we investigated the role of mitochondrial calcium overload and mitochondrial calcium uniporter (MCU) in kidney aging. By comparing the kidney of 2- and 24-month-old mice, we found calcium overload in renal tubular cells of aged kidney, accompanied by significantly elevated expression of MCU. In human proximal renal tubular cell line HK-2, pretreatment with MCU agonist spermine (10 μM) significantly increased mitochondrial calcium accumulation, and induced the production of reactive oxygen species (ROS), leading to renal tubular cell senescence and age-related kidney fibrosis. On the contrary, pretreatment with MCU antagonist RU360 (10 μM) or calcium chelator BAPTA-AM (10 μM) diminished D-gal-induced ROS generation, restored mitochondrial homeostasis, retarded cell senescence, and protected against kidney aging in HK-2 cells. In a D-gal-induced accelerated aging mice model, administration of BAPTA (100 μg/kg. i.p.) every other day for 8 weeks significantly alleviated renal tubuarl cell senescence and fibrosis. We conclude that MCU plays a key role in promoting renal tubular cell senescence and kidney aging. Targeting inhibition on MCU provides a new insight into the therapeutic strategy against kidney aging.










Similar content being viewed by others
Data availability
Transcriptomic data presented in this study can be found at NCBI GEO database (GSE262514).
References
United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2015. 2015; Available from: http://www.un.org/en.
Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009;20:1199–209.
Liu P, Quinn RR, Lam NN, Elliott MJ, Xu Y, James MT, et al. 5-accounting for age in the definition of chronic kidney disease. JAMA Intern Med. 2021;181:1359–66.
Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: Jacc focus seminar. J Am Coll Cardiol. 2020;75:942–51.
Minutolo R, Borrelli S, De Nicola L. Ckd in the elderly: Kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis. 2015;66:184–6.
Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28:2838–44.
O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: Causes and consequences. J Am Soc Nephrol. 2017;28:407–20.
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–53.
Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol. 2017;13:77–89.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9.
Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, et al. Senescent cells: An emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16:718–35.
Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: Relation to aging. Proc Natl Acad Sci USA. 1995;92:258–62.
Petrova NV, Velichko AK, Razin SV, Kantidze OL. Small molecule compounds that induce cellular senescence. Aging Cell. 2016;15:999–1017.
Schmitt R, Melk A. Molecular mechanisms of renal aging. Kidney Int. 2017;92:569–79.
Gallage S, Gil J. Mitochondrial dysfunction meets senescence. Trends Biochem Sci. 2016;41:207–9.
Miwa S, Kashyap S, Chini E, von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 2022;132:e158447.
Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23.
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4.
Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61:654–66.
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23:303–14.
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18:243–58.
Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C, et al. Wnt/β-catenin/ras signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18:e13004.
Martin N, Bernard D. Calcium signaling and cellular senescence. Cell Calcium. 2018;70:16–23.
Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–72.
Manda KR, Tripathi P, Hsi AC, Ning J, Ruzinova MB, Liapis H, et al. Nfatc1 promotes prostate tumorigenesis and overcomes pten loss-induced senescence. Oncogene. 2016;35:3282–92.
Madreiter-Sokolowski CT, Waldeck-Weiermair M, Bourguignon MP, Villeneuve N, Gottschalk B, Klec C, et al. Enhanced inter-compartmental Ca2+ flux modulates mitochondrial metabolism and apoptotic threshold during aging. Redox Biol. 2019;20:458–66.
Madreiter-Sokolowski CT, Thomas C, Ristow M. Interrelation between ros and Ca2+ in aging and age-related diseases. Redox Biol. 2020;36:101678.
Wiel C, Lallet-Daher H, Gitenay D, Gras B, Le Calve B, Augert A, et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat Commun. 2014;5:3792.
Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ros: A mutual interplay. Redox Biol. 2015;6:260–71.
Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–16.
Huang G, Docampo R. The mitochondrial calcium uniporter interacts with subunit c of the atp synthase of trypanosomes and humans. mBio. 2020;11:e00268–20.
Hubbard MJ, McHugh NJ. Mitochondrial atp synthase f1-beta-subunit is a calcium-binding protein. FEBS Lett. 1996;391:323–9.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, atp, and ros: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33.
De Marchi E, Bonora M, Giorgi C, Pinton P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium. 2014;56:1–13.
Yu EPK, Reinhold J, Yu H, Starks L, Uryga AK, Foote K, et al. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol. 2017;37:2322–32.
Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102:893–992.
Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–78.
Marchi S, Corricelli M, Branchini A, Vitto VAM, Missiroli S, Morciano G, et al. Akt-mediated phosphorylation of micu1 regulates mitochondrial Ca2+ levels and tumor growth. EMBO J. 2019;38:e99435.
Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, et al. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–73.
Patron M, Sprenger HG, Langer T. M-aaa proteases, mitochondrial calcium homeostasis and neurodegeneration. Cell Res. 2018;28:296–306.
Li S, Chen J, Liu M, Chen Y, Wu Y, Li Q, et al. Protective effect of hint2 on mitochondrial function via repressing mcu complex activation attenuates cardiac microvascular ischemia-reperfusion injury. Basic Res Cardiol. 2021;116:65.
Ingram AJ, James L, Ly H, Thai K, Scholey JW. Stretch activation of jun n-terminal kinase/stress-activated protein kinase in mesangial cells. Kidney Int. 2000;58:1431–9.
Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The world report on ageing and health: A policy framework for healthy ageing. Lancet. 2016;387:2145–54.
Rudnicka E, Napierala P, Podfigurna A, Meczekalski B, Smolarczyk R, Grymowicz M. The world health organization (who) approach to healthy ageing. Maturitas. 2020;139:6–11.
Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF. Accelerated expression of senescence associated cell cycle inhibitor p16ink4a in kidneys with glomerular disease. Kidney Int. 2007;71:218–26.
Liu J, Yang JR, He YN, Cai GY, Zhang JG, Lin LR, et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin a (IgA) nephropathy. Transl Res. 2012;159:454–63.
Kauppila TES, Kauppila JHK, Larsson NG. Mammalian mitochondria and aging: An update. Cell Metab. 2017;25:57–71.
Luo C, Zhou S, Zhou Z, Liu Y, Yang L, Liu J, et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J Am Soc Nephrol. 2018;29:1238–56.
Ma X, Warnier M, Raynard C, Ferrand M, Kirsh O, Defossez PA, et al. The nuclear receptor rxra controls cellular senescence by regulating calcium signaling. Aging Cell. 2018;17:e12831.
Ziegler DV, Vindrieux D, Goehrig D, Jaber S, Collin G, Griveau A, et al. Calcium channel ITPR2 and mitochondria-ER contacts promote cellular senescence and aging. Nat Commun. 2021;12:720.
Cooper LL, Li W, Lu Y, Centracchio J, Terentyeva R, Koren G, et al. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J Physiol. 2013;591:5895–911.
Ruiz-Meana M, Minguet M, Bou-Teen D, Miro-Casas E, Castans C, Castellano J, et al. Ryanodine receptor glycation favors mitochondrial damage in the senescent heart. Circulation. 2019;139:949–64.
Sileikyte J, Forte M. The mitochondrial permeability transition in mitochondrial disorders. Oxid Med Cell Longev. 2019;2019:3403075.
Calvo-Rodriguez M, Bacskai BJ. Mitochondria and calcium in Alzheimer’s disease: From cell signaling to neuronal cell death. Trends Neurosci. 2021;44:136–51.
Gao P, Jiang Y, Wu H, Sun F, Li Y, He H, et al. Inhibition of mitochondrial calcium overload by Sirt3 prevents obesity- or age-related whitening of brown adipose tissue. Diabetes. 2020;69:165–80.
Kwong JQ, Huo J, Bround MJ, Boyer JG, Schwanekamp JA, Ghazal N, et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight. 2018;3:e121689.
Acknowledgements
This work was supported by National Natural Science Foundation of China (82225010, 82070707); and Guangdong Provincial Clinical Research Center for Kidney Disease (2020B1111170013).
Author information
Authors and Affiliations
Contributions
LLZ conceived the research and designed the experiments. YBX, WYH, XL, SZ, XXW, XLL and LLZ performed the experiments and contributed to acquisition and analysis of data. YBX created the figures and prepared the materials of this study. YBX and LLZ wrote the manuscript. All authors contributed to the article and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
**ong, Yb., Huang, Wy., Ling, X. et al. Mitochondrial calcium uniporter promotes kidney aging in mice through inducing mitochondrial calcium-mediated renal tubular cell senescence. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01298-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01298-5
- Springer Nature Singapore Pte Ltd.