Abstract
Pancreatic ductal adenocarcinoma (PDAC) and cholangiocarcinoma (CCA) are both deadly cancers and they share many biological features besides their close anatomical location. One of the main histological features is neurotropism, which results in frequent perineural invasion. The underlying mechanism of cancer cells favoring growth by and through the nerve fibers is not fully understood. In this review, we provide knowledge of these cancers with frequent perineural invasion. We discuss nerve fiber crosstalk with the main different components of the tumor microenvironment (TME), the immune cells, and the fibroblasts. Also, we discuss the crosstalk between the nerve fibers and the cancer. We highlight the shared signaling pathways of the mechanisms behind perineural invasion in PDAC and CCA. Hereby we have focussed on signaling neurotransmitters and neuropeptides which may be a target for future therapies. Furthermore, we have summarized retrospective results of the previous literature about nerve fibers in PDAC and CCA patients. We provide our point of view in the potential for nerve fibers to be used as powerful biomarker for prognosis, as a tool to stratify patients for therapy or as a target in a (combination) therapy. Taking the presence of nerves into account can potentially change the field of personalized care in these neurotropic cancers.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) and cholangiocarcinoma (CCA) are aggressive cancers with only a limited response to chemotherapy. PDAC mortality is estimated to exceed the total breast, prostate, and colorectal cancer deaths and be the second leading cancer-related death by 2030 [1, 2]. PDAC and CCA share many clinical characteristics, which include high mortality rates and low treatment efficacy [3]. Unfortunately, survival rates have not improved even from recent novel therapeutic targets such as immune checkpoints [3,4,5,6,7]. Biologically PDAC and CCA are characterized by desmoplastic stroma and this stromal compartment is thought to be held responsible for the poor efficacy of chemotherapy. Today, surgery combined with chemotherapy is the only chance of cure [8].
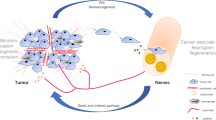
The tumor microenvironment (TME) is a fervent area of research interest as it contains a host of nonmalignant cells that play an important role in carcinogenesis such as fibroblasts, immune cells, blood- and lymphatic vessels, and nerve fibers. In this review, we will focus on the pathways involved in neurogenesis and the interaction between nerve fibers and the other components of the TME.
Internal organs are innervated by the autonomic nervous system (ANS), which is composed of two components: the sympathetic nervous system (SNS) and the parasympathetic nervous system (PSNS). Increasing evidence shows that not only the internal organs are innervated by the PSNS, but solid tumors also depend on the development of nerves in the TME for growth and invasion in adjacent tissue [9,10,11].
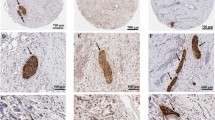
Besides the aggressive behavior and poor response to treatment, another shared feature of these two cancer types is perineural invasion (PNI), which is defined as cancer cells surrounding at least 33% of the epineurial, perineural, and endoneurial space of the nerve sheath [12]. PNI describes the process of cancer cells invading the nerve, crossing all layers of the nerve sheath. Once the cancer cells are invaded in the nerve, they have reached a favorable environment to travel intraneural and contribute to the progression of the disease. Over time different definitions for PNI have been used. It has been described as cancer cells located in the endoneurium associated with the Schwann cells [13] or later on as the presence of cancer cells along one of the layers of the nerve sheath [12, 14,15,16]. For pathologists invasion in one of these nerve sheath layers is used to report PNI and often a mixture of invasion in different layers is seen in one histological slide. Intraneural invasion in PDAC has been associated with higher frequency of local/distant recurrence when compared to cases with PNI but without intraneural invasion [17]. This provides some evidence that defining the level of PNI matters clinically but up to now this is not recommended in the guidelines for the pathologists. The exact underlying mechanism of PNI remains unknown [12, 18]. A hypothesis is that the nerve fibers choose the path of “least resistance” and the cancer cells move along this low resistance path [14, 15]. A recent insight showed that PNI was activating signaling pathways when cancer cells attacked the perineural spaces of the surrounding nerves [12]. Even though PNI commonly occurs in many solid tumors [19,20,21,22], PDAC and CCA are “neurotropic cancers” and have a high frequency of PNI [23]. It has been reported that almost all PDAC lesions contain PNI and about 75% of CCA lesions showed PNI [23,24,25] (Fig. 1 presents the classical PNI pathological characteristics of PDAC and CCA).
Histology slide of PDAC (a) and CCA (b) in Hematoxylin and Eosin (H&E) showing PNI, which is one of the shared pathological characteristics of both cancers. a PDAC slide with extended PNI and an almost identical histomorphology as CCA. b CCA with tumor cells massively invading the perineural space, surrounded by desmoplastic stroma and few small tumor glands in the stroma.
A novel biological phenomenon is the cancer-related neurogenesis, which is described in prostate cancer. The nerve fiber density is increased in paraneoplastic and neoplastic prostate lesions [26]. It is not known whether this cancer-related neurogenesis also occurs in PDAC or CCA. Exploring the role of alterations in nerve fibers in PDAC and CCA has the potential to be of importance in develo** personalized medicine and finding an effective novel treatment strategy.
In this review, we aim to provide an overview of the current knowledge about nerve fiber crosstalk with cancer, and other components of the TME in PDAC and CCA.
Innervation and neurotransmitters
There is a complex nerve fiber network distributed around the pancreas, retroperitoneum and the biliary tree. Nerve fibers in the PDAC TME include axons originating from the sympathetic, parasympathetic, enteropancreatic or hepatic plexus, afferent nerve fibers and newly developed nerve fibers [94]. It was described that some MMPs (MT1-MMP) are shown to degrade the ECM and promote pancreatic cancer expansion, invasion, and progression to an advanced stage [95, 96]. It is reported that MMP9 is associated with more lymph node metastasis and a poorer survival in breast cancer [97]. PDAC cells in vivo undergoing chronic stress, were sensitive to neural signaling and pancreatic stromal cells were increased. This promoted tumor metastasis and cancer progression, β-adrenergic receptor blockade intervention therapy can block this neural signaling and be part of a combination therapy for PDAC [63].
Pathological features of nerve fibers in PDAC and CCA
PNI is considered as an important factor for poor prognosis in PDAC and CCA [24, 98]. It has been shown that PNI can be the reason for curative resection failure (shown in Table 1). Chatterjee et al. showed by examination of 212 PDAC slides, that the presence of PNI was directly correlated with tumor size, margin status, lymph node metastasis, and AJCC stages and inversely associated with disease free survival (DFS) and OS [17]. Shimada et al. found that the degree of intrapancreatic nerve invasion can be used as a predictor for recurrence of disease after surgery [99]. A phenomenon termed as “neural remodeling” is postulated in PDAC, characterized by the alterations in morphology of the nerve [18, 100,101,102]. It was shown that nerve fiber alterations including hypertrophic nerves, increased nerve fiber density and pancreatic neuritis were strongly associated with GAP-43 overexpression and abdominal pain [103]. In the perineural space, PNI induces reactive alterations in the morphology and function of the nerves. Morphological changes include changes of the nerve trunk and thickness [102]. The aggressiveness of PNI is related to neural remodeling, desmoplasia and cancer pain. Severe and enduring pain was strongly associated with poor survival in PDAC patients. However, these neural alterations did not have a significant association with survival [103]. NGF and Artemin play a fundamental role in neural modeling in pancreatic adenocarcinoma.
Interestingly, lower intrapancreatic neural density in the tumor area was linked to shorter OS with multivariate analysis [73]. Related research in CCA mainly focused on PNI prevalence and patient survival [45, 99, 104]. In summary, these studies consistently showed that the presence of PNI was linked to shorter survival in patients with PDAC or CCA. The significance that PNI is an independent prognostic factor of poor outcome has been demonstrated. However, the influence of nerve remodeling especially the new outgrown small nerve fibers has not been fully explained.
Conclusions and future directions
With its frequent PNI, PDAC and CCA are two neurotropic cancers. The neurotropism of these cancers could be an explanation of their aggressiveness and poor response to treatment. In this review, the progress of recent research in the mechanism of PNI in PDAC and CCA is discussed. However, the crosstalk between the nervous system in PDAC and CCA is undiscovered. Different nerve fibers have a different function and the interaction with the components of the TME and the cancer are important to investigate.
In PDAC, the role of nerve fibers is divergent and nerves from the PSNS and SNS have a cancer stimulating and cancer inhibiting effect. To our current knowledge, detailed understanding of the underlying mechanisms of tumor and nerve fiber interaction is critical for the development of innovative therapeutic strategies for patients with these highly lethal cancers. The nerve outgrowth is part of the TME, in which cancer to stroma crosstalk takes place. It is likely that other components of the TME also influence the nerve outgrowth and immune cells and fibroblasts are key components in this process. Targeting nerves has the potential to be a new strategy for therapy for PDAC and CCA patients by influencing the TME, immune cells and fibroblasts, potentially influencing sensitivity to therapeutics. The newly formed nerve fibers are different from the more commonly used PNI. From our perspective, PNI originates in the pre-existing nerve fiber networks and the cancer uses the distribution network for cancer growth. This is a well-known sign of aggressive disease and is associated with poor survival. Small nerve fiber outgrowth can be used as a biomarker for a better survival, as a tool to stratify patients for treatment and as a target for therapeutics. More research is needed to investigate whether sensitivity to already existing immunotherapy can be achieved by targeting nerves.
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–14.
Liu H, Ma Q, Xu Q, Lei J, Li X, Wang Z, et al. Therapeutic potential of perineural invasion, hypoxia and desmoplasia in pancreatic cancer. Curr Pharm Des. 2012;18:2395–403.
Maisonneuve P. Epidemiology and burden of pancreatic cancer. La Presse Médicale. 2019;48:e113–e23.
Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39:19–31.
McClements S, Khan SA. Epidemiology and pathogenesis of cholangiocarcinoma. In: Cross T, Palmer DH, editors. Liver cancers: from mechanisms to management. Cham: Springer International Publishing; 2019. p. 179–86.
Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8:1458–73.
Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surgical Res. 2008;149:319–28.
Zahalka AH, Arnal-Estapé A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6.
Hondermarck H, Jobling P. The sympathetic nervous system drives tumor angiogenesis. Trends Cancer 2018;4:93–4.
March B, Faulkner S, Jobling P, Steigler A, Blatt A, Denham J, et al. Tumour innervation and neurosignalling in prostate cancer. Nat Rev Urol. 2020;17:119–30.
Bapat AA, Galen H, Hoff DD, Von, Haiyong H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695.
Bockman DE, Büchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107:219–30.
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91.
Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94:426–7.
Gasparini G, Pellegatta M, Crippa S, Lena MS, Belfiori G, Doglioni C, et al. Nerves and pancreatic cancer: new insights into a dangerous relationship. Cancers. 2019;11:893.
Chatterjee D, Katz MH, Rashid A, Wang H, Iuga AC, Varadhachary GR, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–17.
Demir IE, Ceyhan GO, Liebl F, D’Haese JG, Maak M, Friess H. Neural invasion in pancreatic cancer: the past, present and future. Cancers. 2010;2:1513–27.
Shen F-Z, Zhang B-Y, Feng Y-J, Jia Z-X, An B, Liu C-C, et al. Current research in perineural invasion of cholangiocarcinoma. J Exp Clin Cancer Res. 2010;29:24.
Liang D, Shi S, Xu J, Zhang B, Qin Y, Ji S, et al. New insights into perineural invasion of pancreatic cancer: more than pain. Biochimica et Biophysica Acta (BBA)—reviews on. Cancer. 2016;1865:111–22.
Pundavela J, Roselli S, Faulkner S, Attia J, Scott RJ, Thorne RF, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626–35.
Zareba P, Flavin R, Isikbay M, Rider JR, Gerke TA, Finn S, et al. Perineural invasion and risk of lethal prostate cancer. Cancer Epidemiol Biomark Prev. 2017;26:719–26.
Chen S-H, Zhang B-Y, Zhou B, Zhu C-Z, Sun L-Q, Feng Y-J. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res. 2019;9:1–21.
Ren K, Yi SQ, Dai Y, Kurosawa K, Miwa Y, Sato I. Clinical anatomy of the anterior and posterior hepatic plexuses, including relations with the pancreatic plexus: a cadaver study. Clin Anat. 2020;33:630–6.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74.
Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–603.
Zuo H-D, Zhang X-M, Li C-J, Cai C-P, Zhao Q-H, **e X-G, et al. CT and MR imaging patterns for pancreatic carcinoma invading the extrapancreatic neural plexus (Part I): anatomy, imaging of the extrapancreatic nerve. World J Radiol. 2012;4:36–43.
Li W, Yu G, Liu Y, Sha L. Intrapancreatic ganglia and neural regulation of pancreatic endocrine secretion. Front Neurosci. 2019;13:21.
Mizuno K, Ueno Y. Autonomic nervous system and the liver. Hepatol Res. 2017;47:160–5.
Rodriguez-Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best Pr Res Clin Endocrinol Metab. 2014;28:745–56.
Babic T, Alberto TR. Neural control of the pancreas. Panc. 2016;27. https://doi.org/10.3998/panc.2016.27.
Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med. 2013;1:27.
Sha M, Cao J, Sun H-y, Tong Y, **a Q. Neuroendocrine regulation of cholangiocarcinoma: a status quo review. Biochim Biophys Acta. 2019;1872:66–73.
Dang N, Meng X, Song H. Nicotinic acetylcholine receptors and cancer. Biomed Rep. 2016;4:515–8.
Liu H-P, Tay S-S-W, Leong S-K, Schemann M. Colocalization of ChAT, DβH and NADPH-d in the pancreatic neurons of the newborn guinea pig. Cell Tissue Res. 1998;294:227–31.
O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–512.
Campbell K, Rossi F, Adams J, Pitsidianaki I, Barriga FM, Garcia-Gerique L, et al. Collective cell migration and metastases induced by an epithelial-to-mesenchymal transition in Drosophila intestinal tumors. Nat Commun. 2019;10:2311.
Barbieri A, Bimonte S, Palma G, Luciano A, Rea D, Giudice A, et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int J Oncol. 2015;47:527–34.
Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75–90.e7.
Kanno N, LeSage G, Phinizy JL, Glaser S, Francis H, Alpini G. Stimulation of α2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329–40.
Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578:449–54.
Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res. 2015;75:1777–81.
Feng Y-J, Zhang B-Y, Yao R-Y, Lu Y. Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells. Hepatobiliary Pancreat Dis Int. 2012;11:418–23.
Zhang L, **u D, Zhan J, He X, Guo L, Wang J, et al. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Onco Targets Ther. 2016;9:6719–26.
Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, et al. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through alpha7nAChR-mediated MUC4 upregulation. Oncogene 2013;32:1384–95.
Amonyingcharoen S, Suriyo T, Thiantanawat A, Watcharasit P, Satayavivad J. Taurolithocholic acid promotes intrahepatic cholangiocarcinoma cell growth via muscarinic acetylcholine receptor and EGFR/ERK1/2 signaling pathway. Int J Oncol. 2015;46:2317–26.
Martínez AK, Jensen K, Hall C, O’Brien A, Ehrlich L, White T, et al. Nicotine promotes cholangiocarcinoma growth in xenograft mice. Am J Pathol. 2017;187:1093–105.
Aloe L, Rocco ML, Balzamino BO, Micera A. Nerve growth factor: role in growth, differentiation and controlling cancer cell development. J Exp Clin Cancer Res. 2016;35:116.
Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer. 2020;20:143–57.
Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–33.
Schecterson LC, Bothwell M. Neurotrophin receptors: old friends with new partners. Dev Neurobiol. 2010;70:332–8.
Molloy NH, Read DE, Gorman AM. Nerve growth factor in cancer cell death and survival. Cancers. 2011;3:510–30.
Yang XQ, Xu YF, Guo S, Liu Y, Ning SL, Lu XF, et al. Clinical significance of nerve growth factor and tropomyosin-receptor-kinase signaling pathway in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2014;20:4076–84.
Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol. 2008;23:1852–9.
Saloman JL, Singhi AD, Hartman DJ, Normolle DP, Albers KM, Davis BM. Systemic depletion of nerve growth factor inhibits disease progression in a genetically engineered model of pancreatic ductal adenocarcinoma. Pancreas. 2018;47:856–63.
Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser SS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–209.
Yue XJ, Xu LB, Zhu MS, Zhang R, Liu C. Over-expression of nerve growth factor-beta in human cholangiocarcinoma QBC939 cells promote tumor progression. PloS One. 2013;8:e62024.
Xu LB, Liu C, Gao GQ, Yu XH, Zhang R, Wang J. Nerve growth factor-beta expression is associated with lymph node metastasis and nerve infiltration in human hilar cholangiocarcinoma. World J Surg. 2010;34:1039–45.
Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219–22.
Eichmann A, Brunet I. Arterial innervation in development and disease. Sci Transl Med. 2014;6:252ps9–ps9.
Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61.
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:713–4.
Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40–7.
Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22:1289–305.
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–44.
Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–41.
Mauffrey P, Tchitchek N, Barroca V, Bemelmans A-P, Firlej V, Allory Y, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569:672–8.
Raju B, Haug SR, Ibrahim SO, Heyeraas KJ. Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience. 2007;149:715–25.
Huang D, Su S, Cui X, Shen X, Zeng Y, Wu W, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine. 2014;93:e172.
Shao JX, Wang B, Yao YN, Pan ZJ, Shen Q, Zhou JY. Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thorac Cancer. 2016;7:588–98.
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115.
Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H, et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117:4834–45.
Iwasaki T, Hiraoka N, Ino Y, Nakajima K, Kishi Y, Nara S, et al. Reduction of intrapancreatic neural density in cancer tissue predicts poorer outcome in pancreatic ductal carcinoma. Cancer Sci. 2019;110:1491–502.
Zhang L, Guo L, Tao M, Fu W, **u D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin J Cancer Res. 2016;28:180–6.
Sinha S, Fu YY, Grimont A, Ketcham M, Lafaro K, Saglimbeni JA, et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 2017;77:1868–79.
Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci USA. 2016;113:3078–83.
Bai H, Li H, Zhang W, Matkowskyj KA, Liao J, Srivastava SK, et al. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis 2011;32:1689–96.
Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR. et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578:449–54.
Soto-Tinoco E, Guerrero-Vargas NN, Buijs RM. Interaction between the hypothalamus and the immune system. Exp Physiol. 2016;101:1463–71.
Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22:72–7.
Mignini F, Sabbatini M, Mattioli L, Cosenza M, Artico M. Neuro-immune modulation of the thymus microenvironment (Review). Int J Mol Med. 2014;33:1392–400.
Al-Shalan HAM, Hu D, Nicholls PK, Greene WK, Ma B. Immunofluorescent characterization of innervation and nerve-immune cell neighborhood in mouse thymus. Cell Tissue Res. 2019;378:239–54.
Veiga-Fernandes H, Pachnis V. Neuroimmune regulation during intestinal development and homeostasis. Nat Immunol. 2017;18:116–22.
Godinho-Silva C, Cardoso F, Veiga-Fernandes H. Neuro-immune cell units: a new paradigm in physiology. Annu Rev Immunol. 2019;37:19–46.
Veiga-Fernandes H, Mucida D. Neuro-immune interactions at barrier surfaces. Cell 2016;165:801–11.
Chesne J, Cardoso V, Veiga-Fernandes H. Neuro-immune regulation of mucosal physiology. Mucosal Immunol. 2019;12:10–20.
Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–97.
Yu M, Tannock IF. Targeting tumor architecture to favor drug penetration: a new weapon to combat chemoresistance in pancreatic cancer? Cancer Cell. 2012;21:327–9.
Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80.
Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–9.
Coulouarn C, Clément B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol. 2014;60:1306–9.
Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226:185–99.
Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci. 2011;3:1165–79.
Ulrich R, Gerhauser I, Seeliger F, Baumgartner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the develo** mouse brain and spinal cord: a reverse transcription quantitative polymerase chain reaction study. Dev Neurosci. 2005;27:408–18.
Maatta M, Soini Y, Liakka A, Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res. 2000;6:2726–34.
Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front Cell Dev Biol. 2019;7:60.
Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–97.
Yamada Y, Mori H, Hijiya N, Matsumoto S, Takaji R, Kiyonaga M, et al. Extrahepatic bile duct cancer: invasion of the posterior hepatic plexuses-evaluation using multidetector CT. Radiology. 2012;263:419–28.
Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N. Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas. 2011;40:464–8.
Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357–61.
Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis INT. 2002;1:469–76.
Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649–59.
Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, et al. Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–86.e1.
Shirai K, Ebata T, Oda K, Nishio H, Nagasaka T, Nimura Y, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2008;32:2395–402.
Fisher SB, Patel SH, Kooby DA, Weber S, Bloomston M, Cho C, et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: a multi-institution analysis. HPB (Oxford). 2012;14:514–22. https://doi.org/10.1111/j.1477-2574.2012.00489.x.
Lenz J, Karasek P, Jarkovsky J, Muckova K, Dite P, Kala Z, et al. Clinicopathological correlations of nestin expression in surgically resectable pancreatic cancer including an analysis of perineural invasion. J Gastrointestin Liver Dis. 2011;20:389–96.
Ceyhan GO, Schäfer K-H, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–31. https://doi.org/10.1097/SLA.0b013e3181d974d4.
Acknowledgements
X. Tan was funded by China Scholarship Council (Grantnumber: 201806210074). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JdeV-G has served as a consultant for AstraZeneca, MSD, and Servier, and has received institutional research funding from Servier. All outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, X., Sivakumar, S., Bednarsch, J. et al. Nerve fibers in the tumor microenvironment in neurotropic cancer—pancreatic cancer and cholangiocarcinoma. Oncogene 40, 899–908 (2021). https://doi.org/10.1038/s41388-020-01578-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01578-4
- Springer Nature Limited
This article is cited by
-
Survival analysis of laparoscopic surgery and open surgery for hilar cholangiocarcinoma: a retrospective cohort study
World Journal of Surgical Oncology (2024)
-
Quantification of perineural invasion in pancreatic ductal adenocarcinoma: proposal of a severity score system
Virchows Archiv (2023)
-
Assessment of the level III of Inoue by preoperative endoscopic ultrasound and elastography: a novel approach to predict a periarterial divestment technique in borderline resectable (BR) or locally advanced (LA) pancreatic adenocarcinoma—How I do it
Langenbeck's Archives of Surgery (2023)
-
The significance of peri-neural invasion in patients with gallbladder carcinoma after curative surgery: a 10 year experience in China
Updates in Surgery (2023)
-
The clinical implications and molecular features of intrahepatic cholangiocarcinoma with perineural invasion
Hepatology International (2023)