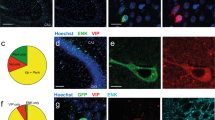
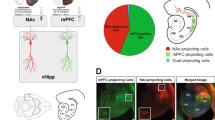
Abstract
A hallmark of the anterior cingulate cortex (ACC) is its functional heterogeneity. Functional and imaging studies revealed its importance in the encoding of anxiety-related and social stimuli, but it is unknown how microcircuits within the ACC encode these distinct stimuli. One type of inhibitory interneuron, which is positive for vasoactive intestinal peptide (VIP), is known to modulate the activity of pyramidal cells in local microcircuits, but it is unknown whether VIP cells in the ACC (VIPACC) are engaged by particular contexts or stimuli. Additionally, recent studies demonstrated that neuronal representations in other cortical areas can change over time at the level of the individual neuron. However, it is not known whether stimulus representations in the ACC remain stable over time. Using in vivo Ca2+ imaging and miniscopes in freely behaving mice to monitor neuronal activity with cellular resolution, we identified individual VIPACC that preferentially activated to distinct stimuli across diverse tasks. Importantly, although the population-level activity of the VIPACC remained stable across trials, the stimulus-selectivity of individual interneurons changed rapidly. These findings demonstrate marked functional heterogeneity and instability within interneuron populations in the ACC. This work contributes to our understanding of how the cortex encodes information across diverse contexts and provides insight into the complexity of neural processes involved in anxiety and social behavior.





Similar content being viewed by others
Data availability
All relevant data are within the paper, and underlying data are available at https://github.com/CruzMartinLab. Custom-written routines for behavioral tracking, Ca2+ imaging analysis, and miniscope models and tools are available at https://github.com/CruzMartinLab. For further information, please contact the corresponding author.
References
Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82.
Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–7.
Lieberman MD, Eisenberger NI. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci USA. 2015;112:15250–5.
Marusak HA, Thomason ME, Peters C, Zundel C, Elrahal F, Rabinak CA. You say ‘prefrontal cortex’ and I say ‘anterior cingulate’: meta-analysis of spatial overlap in amygdala-to-prefrontal connectivity and internalizing symptomology. Transl Psychiatry. 2016;6:e944.
Rolls ET. The cingulate cortex and limbic systems for action, emotion, and memory. Handb Clin Neurol. 2019;166:23–37.
Brockett AT, Tennyson SS, deBettencourt CA, Gaye F, Roesch MR. Anterior cingulate cortex is necessary for adaptation of action plans. Proc Natl Acad Sci USA. 2020;117:6196–204.
Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–10.
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci. 2019;22:1223–34.
Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science 2006;313:1310–2.
Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SE, et al. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc Natl Acad Sci USA. 2014;111:5391–6.
Weible AP, Piscopo DM, Rothbart MK, Posner MI, Niell CM. Rhythmic brain stimulation reduces anxiety-related behavior in a mouse model based on meditation training. Proc Natl Acad Sci USA. 2017;114:2532–7.
Kim SS, Wang H, Li XY, Chen T, Mercaldo V, Descalzi G, et al. Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain. 2011;4:6.
Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J Neurosci. 2009;29:6418–26.
Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG. Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J Neurosci. 2012;32:5598–608.
Weible AP, Rowland DC, Pang R, Kentros C. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J Neurophysiol. 2009;102:2055–68.
**ao Z, Martinez E, Kulkarni PM, Zhang Q, Hou Q, Rosenberg D, et al. Cortical Pain Processing in the Rat Anterior Cingulate Cortex and Primary Somatosensory Cortex. Front Cell Neurosci. 2019;13:165.
Askew CE, Lopez AJ, Wood MA, Metherate R. Nicotine excites VIP interneurons to disinhibit pyramidal neurons in auditory cortex. Synapse. 2019;73:e22116.
Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–70.
Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–4.
Karnani MM, Jackson J, Ayzenshtat I, Hamzehei Sichani A, Manoocheri K, Kim S, et al. Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J Neurosci. 2016;36:3471–80.
Karnani MM, Jackson J, Ayzenshtat I, Tucciarone J, Manoocheri K, Snider WG, et al. Cooperative Subnetworks of Molecularly Similar Interneurons in Mouse Neocortex. Neuron. 2016;90:86–100.
Melzer S, Newmark ER, Mizuno GO, Hyun M, Philson AC, Quiroli E, et al. Bombesin-like peptide recruits disinhibitory cortical circuits and enhances fear memories. Cell 2021;184:5622–34. e25.
Wall NR, De La Parra M, Sorokin JM, Taniguchi H, Huang ZJ, Callaway EM. Brain-Wide Maps of Synaptic Input to Cortical Interneurons. J Neurosci. 2016;36:4000–9.
Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–97.
Poorthuis RB, Enke L, Letzkus JJ. Cholinergic circuit modulation through differential recruitment of neocortical interneuron types during behaviour. J Physiol. 2014;592:4155–64.
Pronneke A, Witte M, Mock M, Staiger JF. Neuromodulation Leads to a Burst-Tonic Switch in a Subset of VIP Neurons in Mouse Primary Somatosensory (Barrel) Cortex. Cereb Cortex. 2020;30:488–504.
Obermayer J, Luchicchi A, Heistek TS, de Kloet SF, Terra H, Bruinsma B, et al. Prefrontal cortical ChAT-VIP interneurons provide local excitation by cholinergic synaptic transmission and control attention. Nat Commun. 2019;10:5280.
Granger AJ, Wang W, Robertson K, El-Rifai M, Zanello AF, Bistrong K, et al. Cortical ChAT(+) neurons co-transmit acetylcholine and GABA in a target- and brain-region-specific manner. Elife. 2020;9.
Garrett M, Manavi S, Roll K, Ollerenshaw DR, Groblewski PA, Ponvert ND, et al. Experience shapes activity dynamics and stimulus coding of VIP inhibitory cells. Elife. 2020;9.
Turi GF, Li WK, Chavlis S, Pandi I, O’Hare J, Priestley JB, et al. Vasoactive intestinal polypeptide-expressing interneurons in the hippocampus support goal-oriented spatial learning. Neuron. 2019;101:1150–65. e8.
Lee AT, Cunniff MM, See JZ, Wilke SA, Luongo FJ, Ellwood IT, et al. VIP interneurons contribute to avoidance behavior by regulating information flow across hippocampal-prefrontal networks. Neuron. 2019;102:1223–34. e4.
Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature. 2018;563:72–8.
Tasic B. Single cell transcriptomics in neuroscience: cell classification and beyond. Curr Opin Neurobiol. 2018;50:242–9.
Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat. 2007;1:3.
Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron. 2016;91:260–92.
Liberti WA 3rd, Markowitz JE, Perkins LN, Liberti DC, Leman DP, Guitchounts G, et al. Unstable neurons underlie a stable learned behavior. Nat Neurosci. 2016;19:1665–71.
Rule ME, Loback AR, Raman DV, Driscoll LN, Harvey CD, O’Leary T Stable task information from an unstable neural population. Elife. 2020;9.
Rule ME, O’Leary T, Harvey CD. Causes and consequences of representational drift. Curr Opin Neurobiol. 2019;58:141–7.
Levy SJ, Kinsky NR, Mau W, Sullivan DW, Hasselmo ME. Hippocampal spatial memory representations in mice are heterogeneously stable. Hippocampus. 2021;31:244–60.
Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD. Dynamic Reorganization of Neuronal Activity Patterns in Parietal Cortex. Cell. 2017;170:986–99. e16.
Chambers AR, Rumpel S. A stable brain from unstable components: Emerging concepts and implications for neural computation. Neuroscience. 2017;357:172–84.
Liberti WA, Perkins LN, Leman DP, Gardner TJ. An open source, wireless capable miniature microscope system. J Neural Eng. 2017;14:045001.
Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013.
Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159.
He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, et al. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron. 2016;92:555.
Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–14.
Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–79.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010;468:270–6.
Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–8.
Aharoni D, Khakh BS, Silva AJ, Golshani P. All the light that we can see: a new era in miniaturized microscopy. Nat Methods. 2019;16:11–3.
Comer AL, Sriram B, Yen WW, Cruz-Martin A A Pipeline using bilateral in utero electroporation to interrogate genetic influences on rodent behavior. J Vis Exp. 2020.
Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 2018;21:1281–9.
Comer AL, **adasa T, Sriram B, Phadke RA, Kretsge LN, Nguyen TPH, et al. Increased expression of schizophrenia-associated gene C4 leads to hypoconnectivity of prefrontal cortex and reduced social interaction. PLoS Biol. 2020;18:e3000604.
Giovannucci A, Friedrich J, Gunn P, Kalfon J, Brown BL, Koay SA, et al. CaImAn an open source tool for scalable calcium imaging data analysis. Elife. 2019;8.
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17:261–72.
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018;97:670–83. e6.
Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, et al. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell. 2017;171:1176–90. e17.
Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, et al. Cortical representations of conspecific sex shape social behavior. Neuron. 2020;107:941–53. e7.
Sriram B, Li L, Cruz-Martin A, Ghosh A. A sparse probabilistic code underlies the limits of behavioral discrimination. Cereb Cortex. 2020;30:1040–55.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76.
Paxinos F The mouse brain in stereotaxic coordinates, 4th Edition. 2012.
Callaway EM, Luo L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci. 2015;35:8979–85.
Murugan M, Jang HJ, Park M, Miller EM, Cox J, Taliaferro JP, et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell. 2017;171:1663–77. e16.
Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74.
Fillinger C, Yalcin I, Barrot M, Veinante P. Afferents to anterior cingulate areas 24a and 24b and midcingulate areas 24a’ and 24b’ in the mouse. Brain Struct Funct. 2017;222:1509–32.
Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–5.
Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J Neurosci. 2017;37:4103–16.
Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 2011;31:4178–87.
Hayden BY, Platt ML. Fool me once, shame on me–fool me twice, blame the ACC. Nat Neurosci. 2006;9:857–9.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22.
Porter BS, Hillman KL. Dorsomedial prefrontal neural ensembles reflect changes in task utility that culminate in task quitting. J Neurophysiol. 2021;126:313–29.
Porter BS, Li K, Hillman KL Regional activity in the rat anterior cingulate cortex and insula during persistence and quitting in a physical-effort task. eNeuro. 2020;7.
Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, Keysers C. Emotional mirror neurons in the rat’s anterior cingulate cortex. Curr Biol. 2019;29:2104.
Porter BS, Hillman KL, Bilkey DK. Anterior cingulate cortex encoding of effortful behavior. J Neurophysiol. 2019;121:701–14.
Hillman KL, Bilkey DK. Persisting through subjective effort: a key role for the anterior cingulate cortex? Behav Brain Sci. 2013;36:691–2. discussion 707-26.
Hillman KL, Bilkey DK. Neurons in the rat anterior cingulate cortex dynamically encode cost-benefit in a spatial decision-making task. J Neurosci. 2010;30:7705–13.
Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr Biol. 2009;19:1532–7.
Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–46.
Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–5.
Krabbe S, Paradiso E, d’Aquin S, Bitterman Y, Courtin J, Xu C, et al. Adaptive disinhibitory gating by VIP interneurons permits associative learning. Nat Neurosci. 2019;22:1834–43.
Letzkus JJ, Wolff SB, Luthi A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron. 2015;88:264–76.
Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84.
Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–20.
Uematsu A, Tan BZ, Johansen JP. Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learn Mem. 2015;22:444–51.
Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–76.
McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988;59:978–96.
Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, et al. Input-specific NMDAR-dependent potentiation of dendritic GABAergic inhibition. Neuron. 2018;97:368–77. e3.
Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–86.
Mank M, Santos AF, Direnberger S, Mrsic-Flogel TD, Hofer SB, Stein V, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5:805–11.
Rose T, Jaepel J, Hubener M, Bonhoeffer T. Cell-specific restoration of stimulus preference after monocular deprivation in the visual cortex. Science. 2016;352:1319–22.
Tolias AS, Ecker AS, Siapas AG, Hoenselaar A, Keliris GA, Logothetis NK. Recording chronically from the same neurons in awake, behaving primates. J Neurophysiol. 2007;98:3780–90.
Margolis DJ, Lutcke H, Schulz K, Haiss F, Weber B, Kugler S, et al. Reorganization of cortical population activity imaged throughout long-term sensory deprivation. Nat Neurosci. 2012;15:1539–46.
Chestek CA, Batista AP, Santhanam G, Yu BM, Afshar A, Cunningham JP, et al. Single-neuron stability during repeated reaching in macaque premotor cortex. J Neurosci. 2007;27:10742–50.
Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009;7:e1000153.
Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–7.
Stevenson IH, Cherian A, London BM, Sachs NA, Lindberg E, Reimer J, et al. Statistical assessment of the stability of neural movement representations. J Neurophysiol. 2011;106:764–74.
Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–95.
Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, et al. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–6.
Rao-Ruiz P, Visser E, Mitric M, Smit AB, van den Oever MC. A Synaptic Framework for the Persistence of Memory Engrams. Front Synaptic Neurosci. 2021;13:661476.
Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007;54:653–66.
Mau W, Hasselmo ME, Cai DJ The brain in motion: How ensemble fluidity drives memory-updating and flexibility. Elife. 2020;9.
Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–803.
Cruz-Martin A, Crespo M, Portera-Cailliau C. Glutamate induces the elongation of early dendritic protrusions via mGluRs in wild type mice, but not in fragile X mice. PLoS One. 2012;7:e32446.
Mostany R, Anstey JE, Crump KL, Maco B, Knott G, Portera-Cailliau C. Altered synaptic dynamics during normal brain aging. J Neurosci. 2013;33:4094–104.
Acknowledgements
We thank all members in the Cruz-Martín lab, as well as Margaret Minnig, Timothy Otchy, Nathan Perkins, and Daniel P. Leman for optimization of miniscope imaging and lens implant placement, Tim Gardner for providing access to 3D printers and sharing video acquisition software, Peyman Golshani and Daniel Aharoni for donating the camera sensor and data acquisition board for miniscope experiments. Ashley Comer, Nancy Padilla, Mark Howe, William A. Liberti 3rd, Michael Hasselmo, Camron Bryant, Renata Batista-Brito, Geoffrey Goodhill and members of the Cruz-Martín lab helped critically by reading the manuscript and engaging in helpful discussions. Todd Blute and the Boston University Biology Imaging Core provided use of the epifluorescence microscope. Lastly, we used the Boston University Shared Computing Cluster to analyze our data. This work was supported by a NARSAD Young Investigator Grant (AC-M, #27202), the NSF NRT UtB: Neurophotonics National Research Fellowship (LNK, #DGE-1633516), and the Boston University Undergraduate Research Opportunities Program (TPHN, WWY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
CJ: Conceptualization: formulated ideas for data analysis (equal), formulated composition and goals of the experiments and paper (equal), Software: code writing and analysis for behavioral and Ca2+imaging data (lead). Formal Analysis: analysis of behavioral and Ca2+ data, statistical analyses (lead). Investigation: surgeries, histology, episcope imaging, rabies cell counting (equal). Validation: validation of Ca2+analysis strategy (equal). Writing—Original Draft: part of methods (support). Writing—Review & Editing: provided edits (equal). Visualization: figure generation (equal). LNK: Conceptualization: formulated composition and goals of the experiments and paper (equal), Software: behavioral analysis with DeepLabCut (equal). Formal Analysis: analysis of behavior, histology, trans-synaptic tracing, c-Fos data, and statistical analyses (equal). Investigation: surgeries and baseplating, behavioral experiments with and without miniscopes, histology, episcope imaging, rabies and c-Fos cell counting (lead). Validation: repeated miniscope experiments with new cohorts (equal). Writing—Original Draft: wrote initial draft (excluding part of methods and discussion) (lead). Writing—Review & Editing: re-wrote draft for revisions and incorporated edits (lead). Visualization: figure generation (equal). WWY Conceptualization: formulated composition and goals of the experiments and paper (equal), Methodology: miniscope adaptation design and construction, developed protocols for GRIN lens implant surgeries (lead). Investigation: surgeries and baseplating, behavioral experiments with miniscopes (equal). Writing—Review & Editing: provided edits (support). Visualization: figure generation (support). KKW Software: behavioral analysis with DeepLabCut (support). Investigation: surgeries, behavioral experiments without miniscopes, immunohistochemistry, histology, episcope imaging, rabies and c-Fos cell counting (equal). BS: Conceptualization: provided ideas for analysis (support). Investigation: set up DeepLabCut with our data (support). Supervision: oversight of some analysis projects (support). Writing—Review & Editing: provided edits (support). AO’C: Software: code writing and analysis of the output of DeepLabCut data (equal). RSL: Software: code writing for Ca2+ imaging analysis (support). Writing—Original Draft: part of methods (support). Visualization: activity heatmaps figure generation (support). JCJ: Methodology: taught us to perform surgeries and use miniscopes (equal). Writing—Review & Editing: provided edits (equal). RAP: Formal Analysis: analysis of behavioral and Ca2+ data (support). Investigation: rabies cell counting and layer quantification (support). CY: Investigation: annotation for behavioral analysis in DeepLabCut, histology, episcope imaging, rabies cell counting (support). TJJ: Formal Analysis: quantification of trans-synaptic tracing data (support). Investigation: surgeries, histology, immunohistochemistry, episcope imaging, cell counting (support). Writing—Review & Editing: provided edits (support). Visualization: figure generation (support). TPHN: Methodology: miniscope adaptation design and construction (equal). ESC: Investigation: annotation for behavioral analysis in DeepLabCut (support). EF: Investigation: surgeries, histology, episcope imaging, rabies cell counting (support). EDS: Investigation: histology, episcope imaging, rabies cell counting (support). BEV: Investigation: rabies cell counting (support). Writing—Review & Editing: provided edits (support). FSH: Investigation: perfusions and histology (support). LAF: Investigation: rabies cell counting and layer quantification (support). Writing—Review & Editing: provided edits (support). AB: Investigation: rabies cell counting (support). SM: Formal Analysis: electrophysiology data analysis (support). Investigation: ex vivo electrophysiology and optogenetic experiments (support). Visualization: figure generation (support). Writing—Review & Editing: feedback for original and revised draft (support). AC-M: Conceptualization: formulated composition, goals, and scope of the paper and approaches for analyses (lead), Formal Analysis: statistical analyses (equal). Writing—Original Draft: wrote some of discussion (equal). Writing—Review & Editing: editing and feedback for original and revised draft (equal). Visualization: figure design and generation (lead). Supervision: mentorship and oversight of the project (lead). Project Administration: management and coordination (lead). Funding acquisition (lead).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Johnson, C., Kretsge, L.N., Yen, W.W. et al. Highly unstable heterogeneous representations in VIP interneurons of the anterior cingulate cortex. Mol Psychiatry 27, 2602–2618 (2022). https://doi.org/10.1038/s41380-022-01485-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01485-y
- Springer Nature Limited