Abstract
Noble hydrogel beads based on gelatin and oxidized corn starch were synthesized, and their usefulness and feasibility in treating effluent with dyes were studied in this work. Tartrazine was introduced as a model material. The kinetics of the tartrazine adsorption of the hydrogel beads were of a pseudo-second-order, and its adsorption isotherm is better fitted with the Langmuir model in comparison with the Freundlich model. With a pH of 2.5 coupled with a stirring speed of 400 r.p.m. and bath temperature of 35 °C, a maximum adsorption capacity of 293 mg g−1 was achieved. The hydrogel beads were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy and X-ray diffraction. The results revealed that the blends have a uniform porous structure and can form stable Schiff base structures. The blend also had good stability and compatibility on mixing with electrolyte solutions, and swelling behavior measurements revealed that the hydrogel beads had excellent water-absorbing capacity.
Similar content being viewed by others
Introduction
The chemical contamination of water is an issue of high concern that influences not only aquatic life and the food web but also inevitably the health of mankind and the sustainable development of society.1 Dyes are a dangerous group of chemicals that are present in the effluents from the leather, food, paper and textile manufacturing industries owing to their complex structure, deep color, high concentrations of organic/inorganic materials, slow biochemical degradation and sustained toxicity.2, 3, 4 Equally alarming is their color change effect and negative impact on water at even very low concentrations. According to reports in the literature, more than 10 000 types of commercial dyes are produced at 0.7–1.0 million tons per year, and approximately 10–15% of synthetic dyes used are discharged into the water system.5, 6 Therefore, it is urgent to develop methods for the treatment of polluted water containing dyes. However, the removal of dyes from wastewater is difficult because of their recalcitrant molecules and complex structure that make them resistant to aerobic digestion and stable to oxidation agents.
The conventional methods of dye removal from effluents involve biological treatment, electrochemical treatment, ion-exchange treatment, membrane filtration process, flocculation, coagulation and/or oxidation.7, 8, 9, 10 However, the high cost and/or inefficiency associated with each of these methods has led to the development of an alternative adsorption method.11, 12 There are many advantages to the adsorption approach, such as its low cost, simplicity of design, ease of operation and insensitivity to toxic substances. Activated carbon, ion-exchange resins, low-cost solid wastes and various synthetic materials are commonly used as absorbents for removing dyes. In recent years, new synthetic materials have been introduced to remove dye from aqueous solutions, with a specific focus on abundantly available, renewable and eco-friendly raw materials.13
Gelatin is one such feasible biomaterial owing to its low cost and high availability. This material is a waste material from the leather industry, obtained via the degradation of solid leather wastes. The rapid dissolution characteristics of gelatin, however, have limited its application in effluent treatments despite its strong dye adsorption potential based on an adsorption reaction between the amino groups in the gelatin and specific groups in the dyes, such as sulfonic acid groups. To reduce its dissolution rate in an aqueous medium, one approach is to use cross-linking, which ‘hardens’ the gelatin.14
In this respect, for such dye adsorption applications, oxidized starch may offer potential as a suitable cross-linking material with gelatin.15 Starch is one of the most abundant and renewable biopolymers in nature.16, 17 Oxidized starch is a reliable and eco-friendly material compared with glutaraldehyde and formaldehyde and can be prepared with different types of functional groups to overcome some of the disadvantages of raw starch. In fact, the cross-linked polysaccharide material by itself exhibits relatively high numbers of amino groups, hydroxyl groups and other functional groups that can effectively remove dyes (especially acid dyes) by adsorption. It is reported that gelatins and oxidized starches can form cross-links through Schiff base formation from a reaction between the ɛ-amino groups of the lysine or hydroxylysine side groups of gelatin and the available aldehydes of the oxidized starch.14 This cross-linking essentially forms a hydrogel. Past reports have shown that such hydrogels can be synthesized in bead form by natural biodegradable materials via irreversible cross-linking in suspension.18
It is noteworthy that hydrogels have been adopted for effluent treatments, as they are three-dimensionally cross-linked polymer networks with a great capacity for water retention that can remain insoluble due to the presence of chemical or physical cross-links.19, 20, 21 Nevertheless, the capability of the hydrogel to remove dyes from aqueous solutions by adsorption remains unclear.22 In particular, there is very little information in the literature on the dye-removal capabilities of such synthetic hydrogel materials utilizing two of the most common natural polymers as adsorbents. The hypothesis of this work was that the cross-linking of oxidized starch with gelatin will form a new synergistic material for dye adsorption applications.
Therefore, the aim of this study was to synthesize oxidized starch–gelatin cross-linked hydrogel beads and to evaluate the usefulness and feasibility of this hydrogel for the removal of dyes from aqueous solutions. Pseudo-first-order and pseudo-second-order models were used to analyze the adsorption kinetic behavior. Tartrazine was introduced as a model dye material as it is widely used in the food industry, leading to discharge into waste streams. The presence of this dye beyond certain thresholds may cause allergy symptoms, commonly including anxiety, migraines, depression, blurred vision, asthma, itching, weakness and a choking sensation.23 It is also considered mutagenic and carcinogenic. One motivation for selecting tartrazine as the model dye material is its highly organic nature and high stability that make conventional physicochemical and biological treatment methods inefficient.
Materials and methods
Materials
Corn starch (moisture content 6.2%, ash content 0.15%, fat content 0.005%, protein content 0.02%) and gelatin were purchased from Sigma-Aldrich in Melbourne, VIC, Australia. To adjust the pH, 0.1 mol l−1 sodium hydroxide (NaOH, Sigma-Aldrich) and 0.1 mol l−1 hydrochloric acid (HCl, Sigma-Aldrich) were used. Tartrazine was also purchased from Sigma-Aldrich in Australia.
Preparation of oxidized corn starch
The oxidized corn starch was prepared as follows. A known concentration (30% wt) of slurry was prepared by mixing corn starch and distilled water with the pH adjusted to 4.0, and the reactants were heated at 80 °C for 1 h with gentle stirring. The temperature of the solution was then kept at 50 °C under continuous stirring, and hydrogen peroxide was added in an amount equal to 30% of the weight of the corn starch. After 6 h, the slurry was immediately separated by high-speed centrifugation, and the obtained oxidized corn starch was dried at 50 °C for 24 h in a vacuum oven.
Preparation of hydrogel beads
Different mass ratios, formulations A to G, for the gelatin and the oxidized corn starch were evaluated, that is, A (10:0), B (8:2), C (6:4), D (5:5), E (4:6), F (2:8), G (0:10), with A and G being pure gelatin and oxidized starch, respectively. The total solute concentrations for all the formulations were maintained at 30 wt%(w/w). The mixed materials were dissolved in distilled water at 80 °C for an initial 30 min at a stirring speed of 100 r.p.m. and subsequently for another 30 min at 500 r.p.m. Seven types of hydrogel beads were obtained by freeze-drying.
Adsorption study
The freeze-dried hydrogel beads with the different formulations described above were soaked in 50 mg g−1 tartrazine aqueous solution with a pH of 2.5 and temperature of 25 °C at a stirring speed of 400 r.p.m. At regular intervals of 30 min, 1 ml of the solution was removed and was centrifuged to determine the tartrazine concentration using ultraviolet spectrometry at a wavelength of 420 nm (Spectra Max M2e, Molecular Devices, Waltham, MA, USA). The concentration of tartrazine corresponding to the intensity of the wavelength measurement was pre-calibrated with known standards of tartrazine. The measurement of the tartrazine concentration was repeatedly undertaken until there was no further change in concentration (at equilibrium). The equilibrium uptake capacity (qe) of tartrazine was then computed using the equation24

where C0 is the initial concentration of tartrazine before adsorption (mg l−1), Ce is the final equilibrium concentration of tartrazine after adsorption (mg l−1), m is the weight of the hydrogel bead adsorbent used (g) and V is the volume of the hydrogel bead adsorbent used (l).
The determined equilibrium uptake capacity was subsequently used in the adsorption kinetics and adsorption isotherm analyses. For the study of the adsorption kinetics, tartrazine solutions with different concentrations (20, 50 and 80 mg g−1) were prepared. For the study of the adsorption isotherm, the concentration was varied between 20 and 300 mg g−1 and the experiments were undertaken at 25, 30 and 35 °C. Similar concentration measurement procedures as described above were adopted for the isotherm experiments.
Characterization of samples
To reveal the internal morphology of the hydrogel beads before and after adsorption, SEM (scanning electron microscopy, Nova Nano SEM, FEI Company, Waltham, MA, USA) micrographs of the freeze-dried samples (before and after adsorption) were obtained at an excitation voltage of 3 kV at different magnifications. The XRD (X-ray diffraction) of corn starch, oxidized corn starch and hydrogel beads before and after adsorption was conducted using a Rigaku Mini Flex 600 diffractometer (Rigaku, Japan) with Ni-filtered Cu Kα(alpha) radiation (40 kV, 15 mA). The scanning range was 10° to 50° with a step size of 2°, and the scanning rate was 2° min−1. The FTIR (Fourier transform infrared spectroscopy, FTIR spectrometer, Perkin-Elmer Company, Waltham, MA, USA) spectra of the corn starch, oxidized corn starch, gelatin and freeze-dried hydrogel beads were also measured.
Swelling behavior of samples
The swelling behavior of the hydrogel beads was studied by determining the increase in weight of the swollen hydrogels at time intervals of 1 h. The dried hydrogel beads (250 mg) were put into a dialysis sack and were kept in an aqueous solution at 37 °C with a stirring speed of 50 r.p.m. in a shaking incubator. At each time interval, the swollen hydrogel beads were taken out and wiped with a filter paper to remove the surface water, weighed and then put back into the same bath.25 The mass determination was continued until the weight of the hydrogel beads remained constant. This experiment was repeated three times for each sample, and the average value was reported. The computation formula for the swelling ratio is

where S is the swelling ratio (%); m0 is the weight of a sample before moisture adsorption; m1 is the weight of a sample after moisture adsorption.
Stability of hydrogel bead solution
Stabilization and compatibility experiments of the hydrogel beads were carried out by mixing with water (hydrogel beads: water=1:9 v/v), salt (NaCl), alkali (NaOH) and acids (H2SO4, HCOOH) at 25 °C for 24 h.
Results and discussion
Effect of gelatin to oxidized starch ratio on the maximum equilibrium uptake capacity
There was no direct correlation between the gelatin to oxidized starch ratio and the equilibrium uptake capacity. The following maximum equilibrium uptake capacity values were obtained for the different formulations: A (120 mg g−1), B (136 mg g−1), C (147 mg g−1), D (165 mg g−1), E (139 mg g−1), F (128 mg g−1), and G (99 mg g−1). The highest maximum uptake capacity was observed at the gelatin to oxidized starch ratio of 5:5. This could be due to the formation of a higher proportion of amorphous region at this ratio. Taking this as the basis, the hydrogel beads with a 5:5 gelatin to oxidized corn starch ratio was used for the subsequent adsorption kinetics and adsorption isotherm experiments.
Adsorption kinetics studies
To investigate the mechanism of adsorption, the rate constants of adsorption for tartrazine were determined using a pseudo-first order equation, which can be expressed as26, 27

where qe is the equilibrium uptake capacity (mg g−1), qt is the amount of solute adsorbed per unit mass of the adsorbent (mg g−1) at time t (min) and k1 is the rate constant (min−1).
Linearized plots of log(qe−qt) against t were used to determine the rate constant (k1) and adsorption at equilibrium (mg g−1 l), which were calculated from the slope and intercept, respectively (Figure 1). R2 indicates the correlation coefficient value for each tartrazine concentration.
The pseudo-second-order equation can be expressed as28

where k2 is the rate constant of pseudo-second-order adsorption (g mg−1min−1). Linearized line plots of  against t are used to obtain rate parameters, as shown in Figure 2. The detailed data are shown in Table 1.
against t are used to obtain rate parameters, as shown in Figure 2. The detailed data are shown in Table 1.
It is clear that under various tartrazine concentrations (20, 50 and 80 mg l−1) the pseudo-second-order equation showed higher correlation coefficients (R2: 0.9888, 0.9988 and 0.9982, respectively) than the pseudo-first-order coefficients (R2: 0.9644, 0.9698 and 0.9869, respectively), indicating that the pseudo-second-order curves have good linearity. The calculated (qe,cal) and experimental values (qe,exp) were entirely different for the pseudo-first-order curves, but the values showed good agreement for the pseudo-second-order curves. Thus, based on Figures 1 and 2 and Table 1, it is clear that the adsorption of tartrazine onto G/OCS beads occurs by a pseudo-second-order adsorption process.
Adsorption isotherms
For the study of the adsorption isotherms, different initial concentrations from 20 to 300 mg g−1 were used at 25, 30 and 35 °C, and the obtained plots are shown in Figure 3. It is evident that higher temperatures lead to an increase in the adsorption equilibrium. Beyond an initial concentration of approximately 80 mg l−1, the equilibrium adsorption capacity ceases to increase and reaches a plateau. This can be further explained by the Langmuir and Freundlich models.
The Freundlich and Langmuir isotherms were used to fit the experimental data. The Langmuir isotherm assumes a surface with homogeneous binding sites with equivalent sorption energies and no interaction between adsorbed species. Mathematically, this can be expressed as29

where qe is the specific metal uptake, Q0 is the maximum adsorption capacity in mg g−1; Ce is the equilibrium concentration in mg l−1; and b represents the affinity of the sorbent for the binding sites, expressed in l mg−1.
The Freundlich model is expressed as

where KF represents the Freundlich constant related to the adsorption capacity, n is the Freundlich constant related to the adsorption intensity, and Ce is the equilibrium concentration of metal ions in the solution.
Figures 4 and 5 show the Langmuir curves and Freundlich curves, respectively. According to these curves, the related data are calculated as shown in Table 2.
These results show that the adsorption at equilibrium increases with the equilibrium concentration and temperature. It can be seen from Table 2 that by the Langmuir model, the maximum adsorption capacity (Q0) increased with the temperature, from 270 (mg g−1; 25 °C) to 294 (mg g−1; 30 °C), and the maximum adsorption capacity was similar to the experimental values in Table 2. The correlation coefficient (R2) was approximately 0.99, which confirms that the adsorption of tartrazine occurred by a process closer to the Langmuir model than to the Freundlich model.
Characteristics of hydrogel beads
Figure 6 shows SEM images of the hydrogel beads before and after the tartrazine adsorption. It is clear that the hydrogel beads have a porous and irregular structure (Figure 6a), and the size of the pores decreased distinctly after the adsorption (Figure 6b). One possible reason is the pressure of the numerous carboxylate anions (COO−) in the network of the hydrogel beads before the adsorption.30, 31 The electrostatic repulsions among the carboxylate anions (COO−) resulted in the expansion of the hydrogel network and an increase in its size, which would produce many small pores.32 After the adsorption, the pore size of the hydrogel beads decreased as a result of the adsorption of the tartrazine, which pushed out the carboxylate anions.
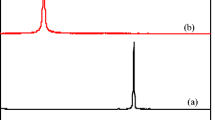
The XRD results of the hydrogel beads before and after adsorption are shown in Figure 7. In Figure 7a, the peaks at 2θ of 15°, 17°, 22°, 23° and 24° are the characteristics peaks of corn starch (CS), which indicate the degree of crystallinity of the corn starch. After oxidation (Figure 7b), the intensities of the peaks at 2θ of 15°, 17° and 22° were much lower than those of corn starch, indicating that the degree of crystallinity of the oxidized corn starch was greatly reduced. At the same time, the peaks at 2θ of 23° and 24° disappeared, indicating the formation of amorphous regions. In Figure 7c, the diffraction signals at 2θ of 23° also disappeared, confirming the presence of a Schiff base between the oxidized corn starch and gelatin, which could destroy the crystallinity of the oxidized starch and expand the amorphous regions in the hydrogel beads. After adsorption (Figure 7d), one new peak can be found at 2θ of 30°, which shows that a new crystalline structure may have formed in the hydrogel, so that it may reasonably be assumed that some functional groups of the hydrogel beads react with tartrazine in aqueous solution.
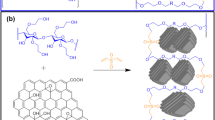
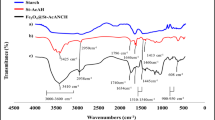
Figure 8 shows the FTIR spectra of the CS, OCS, G and hydrogel beads. In the CS spectrum (curve a), there was a strong absorption peak at approximately 3478 cm−1 that was attributed to the stretching vibration absorption of the hydroxyl groups (O–H) of CS. By comparison with CS, in the OCS (curve b), a new carbonyl group (C=O) vibration characteristic peak appeared at 1736 cm−1. It is concluded that the corn starch has been oxidized and formed OCS. In the G (curve c), the bands at 1689, 1531 and 1290 cm−1 were assigned to the absorption peaks of Amide I, Amide II and Amide III, and the bands at 3373 and 3305 cm−1 were assigned to the absorption peaks of the amide group (N–H). After the OCS was cross-linked with G, the characteristic band for the carbonyl group (C=O) disappeared, and the absorption peaks of Amide I, Amide II and Amide III became weaker and largely disappeared from the infrared spectra of the hydrogel beads (curve d). However, typical bands appeared at 1634 and 1360 cm−1 for the characteristic vibration peaks of C=N and C–N, respectively, in the hydrogel beads (curve d). The results show that the aldehyde groups of OCS had reacted with the amino groups of G and formed stable Schiff base structures.14 In addition, according to the SEM, XRD and FTIR results, the preparation process of the G/OCS hydrogel beads agree wells with Figure 9, which had also shown their adsorption mechanism on tartrazine. When the pH was 2.5, the surface of the G/OCS hydrogel exhibited a positive charge with a large number of hydrogen ions (H+), which would induce a protonation process in the G/OCS hydrogel. On the other hand, the tartrazine molecule could supply a negative charge from the anionic (R′-SO3−), which would attract the positive charges of the G/OCS hydrogel.
The swelling behavior of the hydrogel beads depends on the solubility and the degree of cross-linking. The cross-linking process can be achieved by the Schiff base formation between the aldehyde groups of OCS and amino groups of G. The process of cross-linking improved the stability of the hydrogel beads and generated three-dimensionally cross-linked hydrophilic polymer networks that have the capability to retain a large volume of water in the swollen state.33 OCS and G blends have widespread uses including as absorbent materials, dust suppressants and liquid film mulch. The swelling behavior of the obtained hydrogel beads is illustrated in Figure 10. It can be seen that the swelling degree significantly increased after the OCS was cross-linked with G. The swelling degree of the hydrogel beads could reach 10.5. It can also be seen that the swelling degree of the hydrogel beads significantly increased over time. Moreover, as shown in Figure 10, the swelling degree of the hydrogel beads stabilized at a swelling time of 18 h. It can be concluded that the combination of OCS with G can result in excellent swelling properties.
A high-performance gel solution requires excellent dispersion stability and compatibility. The dispersion stability of a 10% aqueous solutions of hydrogel beads at 50 °C mixed with different materials at 25 °C for 24 h is shown in Table 3. The hydrogel beads exhibited good stability in an acid solution, NaCl solution and alkali solution, which indicates that the compatibility and stability of the blends were improved by mixing OCS with G. This could be because of the active groups in the OCS/G molecules. Because the G has a certain water-solubility, it can increase the water-solubility of the hydrogel bead solution after cross-linking with OCS. In addition, when the acid solution, NaCl solution and alkali solution were added to the hydrogel solution, the acid solution and alkali solution can provide H+ and OH− that can combine with the —NH2 and —COOH, respectively, in the hydrogel bead molecules to form —NH3+ and —COO−, thereby increasing the water-solubility, stability and compatibility. Therefore, the hydrogel beads can be used in an acid, alkali or salt environment without influencing the application effect. Thus, the blend had good stability and compatibility on mixing with electrolyte solutions, which is good for the applications of the blends.
Conclusion
The present study showed that G/OCS hydrogel beads prepared from OCS and G by cross-linking can be used as a promising absorbent for the removal of tartrazine from aqueous solutions. An adsorption kinetics analysis revealed that the adsorption of tartrazine exhibits pseudo-second-order behavior, and the adsorption process obeys the Langmuir model. From a combination of SEM, FTIR and XRD analyses, the G/OCS hydrogel beads have a uniform porous structure and can form stable Schiff base structures. The blend showed good stability and compatibility on mixing with electrolyte solutions, and the swelling behavior measurements also revealed that the hydrogel beads had an excellent water-absorbing capacity. A disadvantage of the G/OCS hydrogel beads was also observed in that a long residence time in an aqueous environment resulted in their partial dissolution, which may limit their recycling capability. In view of the hydrogel beads’ strong potential in dye adsorption, more work is required to further fine-tune the cross-linking process to overcome this limitation.
References
Sun, J. H., Sun, S. P., Wang, G. L. & Qiao, L. P. Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigments 74, 647–652 (2007).
Bilba, D., Suteu, D. & Malutan, T. Removal of reactive dye brilliant red HE-3B from aqueous solutions by hydrolyzed polyacrylonitrile fibres: equilibrium and kinetics modelling. Cent. Eur. J. Chem. 6, 258–266 (2008).
Selvam, P. P., Preethi, S., Basakaralingam, P., Thinakaran, N., Sivasamy, A. & Sivanesan, S. Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J. Harzard. Mater. 155, 39–44 (2008).
Ong, S. T., Lee, C. K. & Zainai, Z. Removal of basic and reactive dyes using ethylenediamine modified rice hull. Biores. Technol. 98, 2792–2799 (2007).
Dizge, N., Aydiner, C., Demirbas, E., Kobya, M. & Kara, S. Adsorption of reactive dyes from aqueous solutions by fly ash: kinetic and equilibrium studies. J. Hazard. Mater. 150, 737–746 (2008).
Dotto, G. L., Vieira, M. L. G. & Pinto, L. A. A. Kinetics and mechanism of tartrazine adsorption onto chitin and chitosan. Ind. Eng. Chem. Res. 51, 6862–6868 (2012).
Mack, C. L., Wilhelmi, B., Duncan, J. R. & Burgess, J. E. A kinetic study of the recovery of platinum ions from an artificial aqueous solution by immobilized Saccharomyces cerevisiae biomass. Minerals Eng. 21, 31–37 (2008).
Kim, T. H., Park, C. & Kim, S. Water recycling from desalination and purification process of reactive dye manufacturing industry by combined membrane filtration. J. Clean. Prod. 13, 779–786 (2005).
Shi, B., Li, G., Wang, D., Feng, C. & Tang, H. Removal of direct dyes by coagulation: the performance of performed polymeric aluminum species. J. Hazard. Mater. 143, 567–574 (2007).
Muruganandham, M. & Swaminathan, R. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigments 69, 196–203 (2006).
Luo, P., Zhang, B., Zhao, Y. F., Wang, J., Zhang, H. & Liu, J. Removal of methylene blue from aqueous solutions by adsorption onto chemically activated halloysite nanotubes. Korean J. Chem. Eng. 28, 800–807 (2011).
Kumar, P. S., Ramalingam, S. & Sathishkumar, K. Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent. Korean J. Chem. Eng. 28, 149–155 (2011).
Shukla, N. B., Rattan, S. & Madras, G. Swelling and dye-adsorption characteristics of an amphoteric superabsorbent polymer. Ind. Eng. Chem. Res. 51, 14941–14948 (2012).
Dang, X., Shan, Z. & Chen, H. Usability of oxidized corn starch-gelatin blends for suppression and prevention of dust. J. Appl. Polym. Sci. 134, 1131–1139 (2016).
Gupta, V. K., Jain, R., Shrivastava, M. & Nayak, A. Equilibrium and thermodynamic studies on the adsorption of the dye tartrazine onto waste ‘coconut husks’ carbon and activated carbon. J. Chem. Eng. Data 55, 5083–5090 (2010).
Moutinho, I. L., Bertges, L. C. & Assis, R. V. Prolonged use of the food dye tartrazine (FD& C yellow no.5) and its effects on the gastric mucosa of wistar rats. Braz. J. Biol. 67, 141–145 (2007).
Zhang, N., Liu, X., Yu, L., Shank, R., Petinaks, E. & Liu, H. Phase composition and interface of starch-gelatin blends studies by synchrotron FTIR micro-spectroscopy. Carbohydr. Polym. 95, 649–653 (2013).
Kaplan, H. & Guner, A. Characterization and determination of swelling and diffusion characteristics of poly(n-vinyl-2-pyrrolidone) hydrogels in water. J. Appl. Polym. Sci. 78, 994–1000 (2000).
Yang, S. P., Fu, S. Y., Liu, H., Zhou, Y. M. & Li, X. Y. Hydrogel beads based on carboxymethyl cellulose for removal heave metal ions. J. Appl. Polym. Sci. 119, 1204–1210 (2011).
Zhang, X. Y., Guo, X. L., Yang, S. G., Tan, S. X., Li, X. F., Dai, H. J., Yu, X. L., Zhang, X. L., Weng, N., Jian, B. & Xu, J. Double-network hydrogel with high mechanical strength prepared from two biocompatible polymers. J. Appl. Polym. Sci. 112, 3063–3070 (2009).
Singh, T. R. S., Carron, P. A. M., Woolfson, A. D. & Donnelly, R. F. Investigation of swelling and network parameters of poly (ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels. Eur. Polym. J. 45, 1239–1249 (2009).
Kahraman, S., Yalcin, P. L. & Kahraman, H. The evaluation of low-cost biosorbents for removal of an azo dye from aqueous solution. Water Environ. J. 26, 399–404 (2012).
Ozay, O., Ekici, S., Baran, Y., Aktas, N. & Sahiner, N. Removal of toxic metal ions with magnetic hydrogels. Water Res. 43, 4403–4411 (2009).
Ngah, W. S. W., Ariff, N. F. M. & Hanafiah, M. A. K. M. Preparation, characterization and environmental application of crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions. Water Air Soil Pollut. 206, 225–236 (2010).
Eid, M., Abdel-Ghaffar, M. A. & Dessouki, A. M. Effect of maleic acid content on the thermal stability, swelling behavior and network structure of gelatin-based hydrogels prepared by gamma irradiation. Nucl. Instrum. Methods Phys. Res. B 267, 91–98 (2009).
Aksu, Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process Biochem. 38, 89–99 (2002).
Donmez, G. & Aksu, Z. Removal of chromium(VI) from saline wastewaters by Dunaliella species. Process Biochem. 38, 751–762 (2002).
Ho, Y. S. Removal of copper ions from aqueous solution by tree fern. Water Res. 37, 2323–2330 (2003).
Rangsayator, N., Upatham, E. S., Kruatrachue, M., Pokethitiyook, P. & Lanza, G. R. Phytoremediation potential of spirulina (arthrospira) platensis: biosorption and toxicity studies of cadmium. Environ. Pollut. 119, 45–53 (2002).
Zhao, Y., Kang, J. & Tan, T. W. Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly(aspartic acid) and poly(acrylic acid). Polymer 47, 7702–7710 (2006).
Ma, J. H., Zhang, L., Fan, B., Xu, Y. J. & Liang, B. A novel sodium carboxymethylcellulose/ poly (N-isopropylacrylamide)/clay semi-IPN nanocomposite hydrogel with improved response rate and mechanical properties. J. Polym. Sci. B Polym. Phys. 46, 1545–1555 (2008).
Ghasemi, S., Mousavi, M. F., Shamsipur, M. & Karami, H. Sonochemical-assisted synthesis of nano-structured lead dioxide. Ultrason. Sonochem. 15, 448 (2008).
Bartil, T., Bounekhel, M., Cedric, C. & Jeerome, R. Swelling behavior and release properties of pH-sensitive hydrogels based on methacrylic derivatives. Acta Pharm. 57, 301–314 (2007).
Acknowledgements
The first author acknowledges the financial support from the China Scholarship Council (CSC student number: 201506240190).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dai, R., Woo, M., Chen, H. et al. Hydrogel beads based on oxidized corn starch cross-linked with gelatin for tartrazine adsorption from aqueous environments. Polym J 49, 549–555 (2017). https://doi.org/10.1038/pj.2017.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.14
- Springer Nature Limited
This article is cited by
-
Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review
Journal of Polymers and the Environment (2023)
-
Aminated magnetic polymeric resin for removal of anthraquinone and azo dyes from aqueous solutions
Journal of Polymer Research (2022)
-
Production and environmental applications of gelatin-based composite adsorbents for contaminants removal: a review
Environmental Chemistry Letters (2021)
-
Freeze-drying of oxidized corn starch: electrochemical synthesis and characterization
Cellulose (2018)