Abstract
Omega-3 polyunsaturated fatty acids are known to have therapeutic potential in several neurological and psychiatric disorders. However, the molecular mechanisms of action underlying these effects are not well elucidated. We previously showed that alpha-linolenic acid (ALA) reduced ischemic brain damage after a single treatment. To follow-up this finding, we investigated whether subchronic ALA treatment promoted neuronal plasticity. Three sequential injections with a neuroprotective dose of ALA increased neurogenesis and expression of key proteins involved in synaptic functions, namely, synaptophysin-1, VAMP-2, and SNAP-25, as well as proteins supporting glutamatergic neurotransmission, namely, V-GLUT1 and V-GLUT2. These effects were correlated with an increase in brain-derived neurotrophic factor (BDNF) protein levels, both in vitro using neural stem cells and hippocampal cultures and in vivo, after subchronic ALA treatment. Given that BDNF has antidepressant activity, this led us to test whether subchronic ALA treatment could produce antidepressant-like behavior. ALA-treated mice had significantly reduced measures of depressive-like behavior compared with vehicle-treated animals, suggesting another aspect of ALA treatment that could stimulate functional stroke recovery by potentially combining acute neuroprotection with long-term repair/compensatory plasticity. Indeed, three sequential injections of ALA enhanced protection, either as a pretreatment, wherein it reduced post-ischemic infarct volume 24 h after a 1-hour occlusion of the middle cerebral artery or as post-treatment therapy, wherein it augmented animal survival rates by threefold 10 days after ischemia.
Similar content being viewed by others
INTRODUCTION
Post-stroke depression (PSD) represents a large portion of the cost spent on the disabilities associated with stroke in the United States (US Department of Health and Human Services, 1999). The estimated prevalence of PSD ranges from 25 to 79% (Gordon and Hibbard, 1997) and is often not detected or inadequately treated (Jia et al, 2006). Stroke patients with PSD suffer higher mortality rates and show only minor improvement in rehabilitation programs, resulting in worse functional (motor and cognitive) outcomes and poorer quality of life (Jorge et al, 2003). Stroke and depression are known to be heterogeneous and multifactorial disorders, but they share common pathological substrates accessible to multitarget strategies. For example, there is increasing evidence that neural plasticity has a key role in both pathologies. Thus, substances that enhance plasticity in the brain could attenuate or reverse their adverse effects and improve the outcome.
In a related disorder, that is, major depressive disorder (MDD), epidemiological and clinical studies suggest that the consumption of omega-3 fatty acids is inversely correlated to the prevalence and the severity of MDD, and that an omega-3 supplementary diet induces benefits in the treatment of depressive states (Hibbeln, 1998; Peet et al, 1998). Thus, omega-3 polyunsaturated fatty acids (PUFA) are good candidates for neuronal protective and restorative strategies against depressive states. In normal mice, chronic administration of a PUFA-supplemented diet consisting of alpha-linolenic acid (ALA), linoleic acid, and oleic acid exerted antidepressant-like effects. These behavioral effects were associated with increases in synaptogenesis, cell number, brain-derived neurotrophic factor (BDNF) gene expression, and hippocampal volume (Venna et al, 2009).
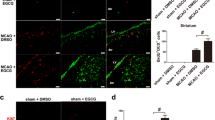
In the domain of stroke disorders, there is growing evidence that the omega-3 precursor, ALA, is a potent protector in several models of global and focal ischemia (McAllister et al, 1995, 1996; Tyler and Pozzo-Miller, 2001; Alonso et al, 2004; Ji et al, 2005), explaining the intense immunostaining for SNAP-25 of the cortical circuitry that we find in the ALA500-treated animals.
The effects of subchronic ALA treatment on increased synaptogenesis and proteins levels involved in exocytosis are in good accordance with recent studies showing that interactions of SNARE proteins with PUFA are of critical importance in regulation of vesicle fusion (Darios et al, 2007; Davletov et al, 2007). As ALA treatment promotes synaptogenesis by modulating the level of SNARE complex, we finally analyzed whether ALA is able to increase the protein levels involved neurotransmitter release. We focused on glutamatergic neurotransmission, whose efficiency is reflected by the expression levels of vesicular glutamate transporters, V-GLUT1 and V-GLUT2. Subchronic ALA treatment induces the upregulation of both V-GLUTs in cortex and hippocampus, suggesting that the omega-3 precursor ALA may promote the neurotransmitter glutamate release. As the cortex and hippocampus are two brain regions known to be crucially involved in synaptic plasticity, our findings suggest that subchronic ALA enhances plastic responses in these two brain regions.
It is plausible that an increase in the number of synapses and/or the upregulation of presynaptic machinery results in ALA–BDNF-potentiation of the neurotransmitter release that could have antidepressant effects. However, while the time course of the various markers of synaptogenesis after ALA treatment are supportive of a functional–anatomical relationship, it is also possible that the behavioral modifications might be due to the production of new neurons rather than modification of existing synapses. As we also showed that ALA might also induce BDNF synthesis by the NSCs, positive synergetic or additive effects have to be considered.
Another part of the present work is regarding the effect of repeated ALA injections in depression. Here, we show that subchronic ALA treatment induces an increase in BDNF levels, and improves neurogenesis and synaptic plasticity in specific brain regions, properties well known for the efficiency of antidepressant drugs (Castren et al, 2007). Using behavioral tests, FST and TST, this work shows that a single ALA injection does not induce antidepressant-like effects, whereas subchronic ALA treatment shows beneficial effects on preventing the development of depression-like behavior in mice in FST and TST paradigms. These results are in good accordance with the antidepressant-like effects of omega 3-enriched diet (Carlezon et al, 2005; Huang et al, 2008; Venna et al, 2009). Furthermore, the subchronic ALA treatment offers a shorter duration of treatment, as compared with diet and a long-lasting antidepressant-like effect, which is still observed 3 days after the final administered dose. It is known that antidepressant drugs have a positive effect on PSD by mediating the signaling pathways of brain plasticity (Santarelli et al, 2003; Dranovsky and Hen, 2006). Neurogenesis and synaptogenesis associated with subchronic ALA treatment are strong arguments in favor of such mechanisms involved in the ALA-antidepressant effect, which can also be related to additive/or synergic interactions with serotonin, norepinephrine, or dopamine pathways (Delion et al, 1994, 1996).
With regard to stroke and long-lasting efficiency of ALA on antidepressant behavior and neuroplasticity, we have independently tested whether a protection against ischemia will be observed 3 days after the final administered dose. Our results show that the subchronic ALA treatment is efficient as preventive treatment. The several protective effects of ALA on neurons (including reduction of excitotoxicity, neurotrophic factor induction, and microenvironmental-associated plasticity changes) may be difficult to interpret how neurogenesis, produced by subchronic ALA treatment before MCAO, reduces infarct volume. In contrast, it seems intuitive that post-MCAO, subchronic ALA treatment may restore neuronal loss, in part, by promoting neurogenesis. In addition, neurogenesis could affect the post-stroke recovery period, which represents yet another target for more efficient therapeutic effects. ALA treatment can be perceived as a ‘restorative’ intervention, which can explain the observed reduced mortality.
In this work, we show that subchronic ALA treatment enhances endogenous neurogenesis and synaptogenesis by a neuronal pathway. Given our previous findings of vasoactive ALA-properties (Blondeau et al, 2007) and increases in neurogenesis being associated with vascular changes (Palmer et al, 2000), we cannot exclude a facilitation of neurogenesis and synaptogenesis related to a vasculature-mediated effect of subchronic ALA treatment.
In summary, the present work shows that a subchronic treatment with ALA injections promotes several features associated with neurogenesis/synaptogenesis and possesses antidepressant-like qualities that may be beneficial for stroke recovery. This functional benefit may be related to acute increased vasodilation and excitotoxicity prevention, as well as long-term enhancement of neurogenesis, synaptogenesis, and neurotransmitter transmission. From a clinical point of view, this ‘multitarget’ effect of subchronic ALA treatment may represent a novel approach to stroke and depression. This multitargeted strategy may extend to other neurodegenerative and psychiatric disorders.
References
Alonso M, Medina JH, Pozzo-Miller L (2004). ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem 11: 172–178.
Alsina B, Vu T, Cohen-Cory S (2001). Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci 4: 1093–1101.
Barde YA (1994). Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res 390: 45–56.
Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R et al (2007). Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res 101: 176–184.
Blondeau N, Widmann C, Lazdunski M, Heurteaux C (2001). Activation of the nuclear factor-kappaB is a key event in brain tolerance. J Neurosci 21: 4668–4677.
Blondeau N, Widmann C, Lazdunski M, Heurteaux C (2002). Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience 109: 231–241.
Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C et al (2007). Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE 2: e373.
Carlezon Jr WA, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF (2005). Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry 57: 343–350.
Castren E, Voikar V, Rantamaki T (2007). Role of neurotrophic factors in depression. Curr Opin Pharmacol 7: 18–21.
Chopp M, Li Y, Zhang ZG (2009). Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 40: S143–S145.
Darios F, Connell E, Davletov B (2007). Phospholipases and fatty acid signalling in exocytosis. J Physiol 585: 699–704.
Davletov B, Connell E, Darios F (2007). Regulation of SNARE fusion machinery by fatty acids. Cell Mol Life Sci 64: 1597–1608.
Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G (1996). alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem 66: 1582–1591.
Delion S, Chalon S, Herault J, Guilloteau D, Besnard JC, Durand G (1994). Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr 124: 2466–2476.
Dranovsky A, Hen R (2006). Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59: 1136–1143.
Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M et al (2006). Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 67: 1954–1967.
Fremeau Jr RT, Voglmaier S, Seal RP, Edwards RH (2004). VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 27: 98–103.
Golanov EV, Reis DJ (1995). Contribution of cerebral edema to the neuronal salvage elicited by stimulation of cerebellar fastigial nucleus after occlusion of the middle cerebral artery in rat. J Cereb Blood Flow Metab 15: 172–174.
Gordon WA, Hibbard MR (1997). Poststroke depression: an examination of the literature. Arch Phys Med Rehabil 78: 658–663.
Hepp R, Perraut M, Chasserot-Golaz S, Galli T, Aunis D, Langley K et al (1999). Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia 27: 181–187.
Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M et al (2004). TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J 23: 2684–2695.
Heurteaux C, Laigle C, Blondeau N, Jarretou G, Lazdunski M (2006a). Alpha-linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience 137: 241–251.
Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD et al (2006b). Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci 9: 1134–1141.
Hibbeln JR (1998). Fish consumption and major depression. Lancet 351: 1213.
Huang EJ, Reichardt LF (2001). Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736.
Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP (2008). Omega-3 fatty acids on the forced-swimming test. J Psychiatr Res 42: 58–63.
Iversen L (2006). Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol 147 (Suppl 1): S82–S88.
Jahn R, Lang T, Sudhof TC (2003). Membrane fusion. Cell 112: 519–533.
Jeftinija SD, Jeftinija KV, Stefanovic G (1997). Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res 750: 41–47.
Ji Y, Pang PT, Feng L, Lu B (2005). Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci 8: 164–172.
Jia H, Damush TM, Qin H, Ried LD, Wang X, Young LJ et al (2006). The impact of poststroke depression on healthcare use by veterans with acute stroke. Stroke 37: 2796–2801.
Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM (2005). The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem 94: 713–722.
Jorge RE, Robinson RG, Arndt S, Starkstein S (2003). Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry 160: 1823–1829.
Knaus P, Betz H, Rehm H (1986). Expression of synaptophysin during postnatal development of the mouse brain. J Neurochem 47: 1302–1304.
Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C (2003). Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg 38: 564–575.
Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M (2000). Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 19: 1784–1793.
Leclerc N, Beesley PW, Brown I, Colonnier M, Gurd JW, Paladino T et al (1989). Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol 280: 197–212.
Lemaire V, Koehl M, Le Moal M, Abrous DN (2000). Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97: 11032–11037.
Lipsky RH, Marini AM (2007). Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann NY Acad Sci 1122: 130–143.
Lu B, Figurov A (1997). Role of neurotrophins in synapse development and plasticity. Rev Neurosci 8: 1–12.
Marini AM, Popolo M, Pan H, Blondeau N, Lipsky RH (2008). Brain adaptation to stressful stimuli: a new perspective on potential therapeutic approaches based on BDNF and NMDA receptors. CNS Neurol Disord Drug Targets 7: 382–390.
Mattson MP, Duan W, Guo Z (2003). Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem 84: 417–431.
Maysami S, Lan JQ, Minami M, Simon RP (2008). Proliferating progenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab 28: 1104–1113.
McAllister AK, Katz LC, Lo DC (1996). Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17: 1057–1064.
McAllister AK, Lo DC, Katz LC (1995). Neurotrophins regulate dendritic growth in develo** visual cortex. Neuron 15: 791–803.
Montgomery P, Richardson AJ (2008). Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst Rev 2: Art no: CD005169.
Naylor M, Bowen KK, Sailor KA, Dempsey RJ, Vemuganti R (2005). Preconditioning-induced ischemic tolerance stimulates growth factor expression and neurogenesis in adult rat hippocampus. Neurochem Int 47: 565–572.
Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2005). I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136: 161–169.
Owen C, Rees AM, Parker G (2008). The role of fatty acids in the development and treatment of mood disorders. Curr Opin Psychiatry 21: 19–24.
Pallini R, Vitiani LR, Bez A, Casalbore P, Facchiano F, Di Giorgi Gerevini V et al (2005). Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery 57: 1014–1025; discussion 1014–1025.
Palmer TD, Willhoite AR, Gage FH (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479–494.
Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG (1995). Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett 377: 489–492.
Peet M, Murphy B, Shay J, Horrobin D (1998). Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 43: 315–319.
Porsolt RD, Bertin A, Jalfre M (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229: 327–336.
Racagni G, Popoli M (2008). Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci 10: 385–400.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809.
Schabitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S (2000). Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 31: 2212–2217.
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376.
Sudhof TC, De Camilli P, Niemann H, Jahn R (1993). Membrane fusion machinery: insights from synaptic proteins. Cell 75: 1–4.
Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395: 347–353.
Takai N, Sasaoka K, Inoue K, Takahashi M, Endo Y, Hatanaka H (1997). Brain-derived neurotrophic factor increases the stimulation-evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J Neurochem 68: 370–375.
Tang X, Falls DL, Li X, Lane T, Luskin MB (2007). Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. J Neurosci 27: 5837–5844.
Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B (2001). Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem 276: 37585–37593.
Tyler WJ, Pozzo-Miller LD (2001). BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249–4258.
U.S. Dept of Health and Human Services (1999). Mental Health: A report of the Surgeon General. Rockville, MD: U.S. Dept of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, NIH, NIMH.
Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R (2009). PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 34: 199–211.
Wada T, Haigh JJ, Ema M, Hitoshi S, Chaddah R, Rossant J et al (2006). Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci 26: 6803–6812.
Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H (2004). Localization of SNARE proteins and secretory organelle proteins in astrocytes in vitro and in situ. Neurosci Res 48: 249–257.
**ao YF, Wright SN, Wang GK, Morgan JP, Leaf A (1998). Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proc Natl Acad Sci USA 95: 2680–2685.
Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N et al (2002). Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci 22: 7580–7585.
Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C et al (2001). FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci USA 98: 5874–5879.
Zhang Y, Pardridge WM (2001). Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood-brain barrier drug targeting system. Stroke 32: 1378–1384.
Acknowledgements
The authors are grateful to Dr Thierry Coppola for his helpful advice on the synaptogenesis portion of this work. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Groupe Lipides et Nutrition (GLN), ONIDOL, The Fondation pour la Recherche Médicale (FRM—allocation jeune équipe), the Fondation Coeur et Artères, the Fond National de la Recherche Scientifique de Belgique, and the Defense Brain and Spinal Cord Injury Program (DBSCIP) (HU-0001-06-0005).
Author information
Authors and Affiliations
Corresponding author
Additional information
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Blondeau, N., Nguemeni, C., Debruyne, D. et al. Subchronic Alpha-Linolenic Acid Treatment Enhances Brain Plasticity and Exerts an Antidepressant Effect: A Versatile Potential Therapy for Stroke. Neuropsychopharmacol 34, 2548–2559 (2009). https://doi.org/10.1038/npp.2009.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2009.84
- Springer Nature Switzerland AG
Keywords
This article is cited by
-
Neuroprotective effect of chia seed oil nanoemulsion against rotenone induced motor impairment and oxidative stress in mice model of Parkinson’s disease
Advances in Traditional Medicine (2023)
-
Diabetes Exacerbates Sepsis-Induced Neuroinflammation and Brain Mitochondrial Dysfunction
Inflammation (2022)
-
A Walnut Diet in Combination with Enriched Environment Improves Cognitive Function and Affects Lipid Metabolites in Brain and Liver of Aged NMRI Mice
NeuroMolecular Medicine (2021)
-
Walnut-Associated Fatty Acids Inhibit LPS-Induced Activation of BV-2 Microglia
Inflammation (2020)
-
Alpha-Linolenic Acid Treatment Reduces the Contusion and Prevents the Development of Anxiety-Like Behavior Induced by a Mild Traumatic Brain Injury in Rats
Molecular Neurobiology (2018)