Abstract
Most tumors frequently undergo initial treatment with a chemotherapeutic agent but ultimately develop resistance, which limits the success of chemotherapies. As cisplatin exerts a high therapeutic effect in a variety of cancer types, it is often used in diverse strategies, such as neoadjuvant, adjuvant and combination chemotherapies. However, cisplatin resistance has often manifested regardless of cancer type, and it represents an unmet clinical need. Since we found that API5 expression was positively correlated with chemotherapy resistance in several specimens from patients with cervical cancer, we decided to investigate whether API5 is involved in the development of resistance after chemotherapy and to explore whether targeting API5 or its downstream effectors can reverse chemo-resistance. For this purpose, cisplatin-resistant cells (CaSki P3 CR) were established using three rounds of in vivo selection with cisplatin in a xenografted mouse. In the CaSki P3 CR cells, we observed that API5 acted as a chemo-resistant factor by rendering cancer cells resistant to cisplatin-induced apoptosis. Mechanistic investigations revealed that API5 mediated chemo-resistance by activating FGFR1 signaling, which led to Bim degradation. Importantly, FGFR1 inhibition using either an siRNA or a specific inhibitor disrupted cisplatin resistance in various types of API5high cancer cells in an in vitro cell culture system as well as in an in vivo xenograft model. Thus, our results demonstrated that API5 promotes chemo-resistance and that targeting either API5 or its downstream FGFR1 effectors can sensitize chemo-refractory cancers.
Similar content being viewed by others
Introduction
In general, traditional therapies such as surgery, chemotherapy and radiotherapy are implemented to treat many cancer types. Among these, chemotherapy is frequently used in diverse strategies, including neoadjuvant, adjuvant and combination chemotherapies.1 Many patients with different types of cancers (for example, cervical, head and neck, non-small cell lung, gastric and bladder cancer) have been preferentially treated with cisplatin chemotherapy.2, 3, 25 These findings suggest the possibility that the effect of chemo-radiation therapy can be compromised in cervical cancer patients with API5 overexpression, an observation that can have profound clinical implications.
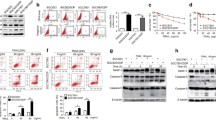
Here our study provided further evidence to satisfy an unmet clinical need. We elucidated the specific molecular mechanism for the close correlation between API5 overexpression and acquired resistance to cisplatin treatment. At first, the tumor cell population with high API5 expression levels steadily increased during the process of establishing a therapeutic resistance model using cisplatin (Figure 1). This result indicated that API5-overexpressing tumor cells are selected by repeated cisplatin treatments and develop resistance to this drug. There is evidence regarding the intimate relationship between API5 and cisplatin resistance. In addition, the crucial API5/FGFR/ERK axis that confers cisplatin resistance provides important clues suggesting that targeting molecules within the API5 downstream pathway can help overcome cisplatin resistance.
API5 contains protein-protein interaction domains such as HEAT and ARM repeats that are well suited for interactions with multiple binding partners.28 Thus, discovering an API5 inhibitor using structure-based drug design studies is possible. However, there are no small-molecule drugs that directly target API5 to date. Considering this, we took note of an inhibitor of API5-mediated FGFR activity, which is a receptor tyrosine kinase that is part of a major class of drug targets, as a potential inhibitor of API5 signaling.29, 30, 31, 32 In a recent report, the small-molecule allosteric inhibitor SSR128129E, which blocks FGFR signaling at nanomolar doses, was identified,33 and its therapeutic potential has been verified in immuno-resistant cancer cells.34 It was determined whether this drug can attenuate the API5-mediated resistant properties induced by cisplatin in various types of aggressive cancer cells with high levels of API5 expression. The results were as predicted—when SSR128129E was used in combination with cisplatin in cisplatin-resistant API5high cells, we observed that the cisplatin-mediated resistant phenotype disappeared. The observed synergistic effect of the combination treatment with cisplatin and SSR128129E is shown in Figure 5. This phenomenon may ultimately be driven by a dramatic increase in caspase 3 and Bim levels. Several studies have shown that cisplatin sensitivity is mediated by either Bim or caspase 3 regulation.35, 36 Thus, our results can be interpreted as a convergence of necrotic features via accumulation of cisplatin-induced apoptotic signals in cells with increased sensitivity to cisplatin via inhibition of FGFR signaling by SSR128129E.
Clinically, a therapeutic strategy with cisplatin that is effective against multiple types of cancers is necessary to minimize cisplatin resistance. In our study, we also conducted in vivo experiments to verify the potential of clinical application of this novel therapeutic strategy. We confirmed that SSR128129E significantly increased the therapeutic efficacy in an established xenograft mouse model using cisplatin-resistant human cancer cells when SSR128129E was used in combination with cisplatin. Thus, we consider it appropriate that during cisplatin-based chemotherapy, FGFR-targeted strategies are useful to treat refractory cancers as well as cisplatin-resistant cancers with high levels of API5 expression.
References
DeVita VT Jr, Chu E . A history of cancer chemotherapy. Cancer Res 2008; 68: 8643–8653.
Florea AM, Busselberg D . Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011; 3: 1351–1371.
Shah N, Dizon DS . New-generation platinum agents for solid tumors. Future Oncol 2009; 5: 33–42.
Zhang J, Wang L, **ng Z, Liu D, Sun J, Li X et al. Status of bi- and multi-nuclear platinum anticancer drug development. Anticancer Agents Med Chem 2010; 10: 272–282.
Rosenberg B, Vancamp L, Krigas T . Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965; 205: 698–699.
Chen D, Milacic V, Frezza M, Dou QP . Metal complexes, their cellular targets and potential for cancer therapy. Curr Pharm Des 2009; 15: 777–791.
Sherman SE, Gibson D, Wang AH, Lippard SJ . X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt(NH3)2(d(pGpG))]. Science 1985; 230: 412–417.
Eastman A . Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells 1990; 2: 275–280.
Jordan P, Carmo-Fonseca M . Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci 2000; 57: 1229–1235.
Siddik ZH . Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003; 22: 7265–7279.
Brabec V, Kasparkova J . Modifications of DNA by platinum complexes. Relation to resistance of tumors to platinum antitumor drugs. Drug Resist Updat 2005; 8: 131–146.
Sedletska Y, Giraud-Panis MJ, Malinge JM . Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents 2005; 5: 251–265.
Kartalou M, Essigmann JM . Mechanisms of resistance to cisplatin. Mutat Res 2001; 478: 23–43.
Ralph SJ, Neuzil J . Mitochondria as targets for cancer therapy. Mol Nutr Food Res 2009; 53: 9–28.
Park CM, Park MJ, Kwak HJ, Moon SI, Yoo DH, Lee HC et al. Induction of p53-mediated apoptosis and recovery of chemosensitivity through p53 transduction in human glioblastoma cells by cisplatin. Int J Oncol 2006; 28: 119–125.
**e SY, Li YJ, Wang PY, Jiao F, Zhang S, Zhang WJ . miRNA-regulated expression of oncogenes and tumor suppressor genes in the cisplatin-inhibited growth of K562 cells. Oncol Rep 2010; 23: 1693–1700.
Shen DW, Pouliot LM, Hall MD, Gottesman MM . Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012; 64: 706–721.
Dai Y, ** S, Li X, Wang D . The involvement of Bcl-2 family proteins in AKT-regulated cell survival in cisplatin resistant epithelial ovarian cancer. Oncotarget 2017; 8: 1354–1368.
Noh KH, Kim SH, Kim JH, Song KH, Lee YH, Kang TH et al. API5 confers tumoral immune escape through FGF2-dependent cell survival pathway. Cancer Res 2014; 74: 3556–3566.
Noh KH, Kang TH, Kim JH, Pai SI, Lin KY, Hung CF et al. Activation of Akt as a mechanism for tumor immune evasion. Mol Ther 2009; 17: 439–447.
Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest 2012; 122: 4077–4093.
Noh KH, Lee YH, Jeon JH, Kang TH, Mao CP, Wu TC et al. Cancer vaccination drives Nanog-dependent evolution of tumor cells toward an immune-resistant and stem-like phenotype. Cancer Res 2012; 72: 1717–1727.
Mao CP, Wu T, Song KH, Kim TW . Immune-mediated tumor evolution: Nanog links the emergence of a stem like cancer cell state and immune evasion. Oncoimmunology 2014; 3: e947871.
Rigou P, Piddubnyak V, Faye A, Rain JC, Michel L, Calvo F et al. The antiapoptotic protein AAC-11 interacts with and regulates Acinus-mediated DNA fragmentation. EMBO J 2009; 28: 1576–1588.
Cho H, Chung JY, Song KH, Noh KH, Kim BW, Chung EJ et al. Apoptosis inhibitor-5 overexpression is associated with tumor progression and poor prognosis in patients with cervical cancer. BMC Cancer 2014; 14: 545.
Song KH, Kim SH, Noh KH, Bae HC, Kim JH, Lee HJ et al. Apoptosis inhibitor 5 increases metastasis via Erk-mediated MMP expression. BMB Rep 2015; 48: 330–335.
Han HD, Song CK, Park YS, Noh KH, Kim JH, Hwang T et al. A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. Int J Pharm 2008; 350: 27–34.
Han BG, Kim KH, Lee SJ, Jeong KC, Cho JW, Noh KH et al. Helical repeat structure of apoptosis inhibitor 5 reveals protein-protein interaction modules. J Biol Chem 2012; 287: 10727–10737.
Overington JP, Al-Lazikani B, Hopkins AL . How many drug targets are there? Nat Rev Drug Discov 2006; 5: 993–996.
Bono F, De Smet F, Herbert C, De Bock K, Georgiadou M, Fons P et al. Inhibition of tumor angiogenesis and growth by a small-molecule multi-FGF receptor blocker with allosteric properties. Cancer Cell 2013; 23: 477–488.
Gasparini G, Longo R, Toi M, Ferrara N . Angiogenic inhibitors: a new therapeutic strategy in oncology. Nat Clin Pract Oncol 2005; 2: 562–577.
Zhang J, Yang PL, Gray NS . Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 2009; 9: 28–39.
Herbert C, Schieborr U, Saxena K, Juraszek J, De Smet F, Alcouffe C et al. Molecular mechanism of SSR128129E, an extracellularly acting, small-molecule, allosteric inhibitor of FGF receptor signaling. Cancer Cell 2013; 23: 489–501.
Song KH, Cho H, Kim S, Lee HJ, Oh SJ, Woo SR et al. API5 confers cancer stem cell-like properties through the FGF2-NANOG axis. Oncogenesis 2017; 6: e285.
Wang J, Zhou JY, Wu GS . Bim protein degradation contributes to cisplatin resistance. J Biol Chem 2011; 286: 22384–22392.
Henkels KM, Turchi JJ . Cisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell lines. Cancer Res 1999; 59: 3077–3083.
Acknowledgements
This work was funded by the National Research Foundation of Korea (NRF-2014R1A2A1A10054205, NRF-2013M3A9D3045881 and NRF-2013R1A1A2063031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jang, H., Woo, S., Song, KH. et al. API5 induces cisplatin resistance through FGFR signaling in human cancer cells. Exp Mol Med 49, e374 (2017). https://doi.org/10.1038/emm.2017.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2017.130
- Springer Nature Limited
This article is cited by
-
Altered expression of anti-apoptotic protein Api5 affects breast tumorigenesis
BMC Cancer (2023)
-
Endometrial cancer PDX-derived organoids (PDXOs) and PDXs with FGFR2c isoform expression are sensitive to FGFR inhibition
npj Precision Oncology (2023)
-
TRPV1 inhibition overcomes cisplatin resistance by blocking autophagy-mediated hyperactivation of EGFR signaling pathway
Nature Communications (2023)
-
FGFR Inhibitors: Clinical Activity and Development in the Treatment of Cholangiocarcinoma
Current Oncology Reports (2021)