Abstract
Visualization of specific genomic loci in live cells is a prerequisite for the investigation of dynamic changes in chromatin architecture during diverse biological processes, such as cellular aging. However, current precision genomic imaging methods are hampered by the lack of fluorescent probes with high specificity and signal-to-noise contrast. We find that conventional transcription activator-like effectors (TALEs) tend to form protein aggregates, thereby compromising their performance in imaging applications. Through screening, we found that fusing thioredoxin with TALEs prevented aggregate formation, unlocking the full power of TALE-based genomic imaging. Using thioredoxin-fused TALEs (TTALEs), we achieved high-quality imaging at various genomic loci and observed aging-associated (epi) genomic alterations at telomeres and centromeres in human and mouse premature aging models. Importantly, we identified attrition of ribosomal DNA repeats as a molecular marker for human aging. Our study establishes a simple and robust imaging method for precisely monitoring chromatin dynamics in vitro and in vivo.
Similar content being viewed by others
Introduction
In the human nuclear genome, ∼3.2 billion base pairs of DNA are tightly packed into 23 chromosome pairs of varying size. Although we can obtain sequence information for any genomic locus with ease, we are still in the early stages of unraveling the organization of the human genome and the spatiotemporal relationships between genomic loci in three-dimensional (3D), which will consequently improve our understanding of genetic and epigenetic regulation during cell differentiation, aging, and pathophysiological processes1,2,3,4,5,Full size image
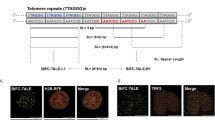
TALEs are composed of multiple highly repetitive modules, a feature that likely predisposes them to self-assemble into bulky protein aggregates especially when being simultaneously tethered to multiple copies of genomic repetitive DNA sequences, preventing their binding to cognate DNA sequences. We thus screened a panel of peptides known to facilitate expression of insoluble proteins in Escherichia coli30, and fused them with TALEs (Figure 2A). We used the following fusion partners for initial screening: ubiquitin (UB), small ubiquitin-related modifier (SUMO), glutathione S-transferase (GST), maltose-binding protein (MBP), and thioredoxin (TRX) (Figure 2B and 2C). Among all peptides tested in telomeres, TRX was the best candidate, as the use of TRX-fused TALEtelo (TTALEtelo) led to nearly perfect co-localization of telomeric FISH and TTALEtelo signals (Figure 2B and 2C). Likewise, the TRX-fused TALEcentro (TTALEcentro) also yielded specific signals precisely marking centromeres and overlap** with centromeric FISH signals (Figure 3A-3C) as well as the centromeric protein CENPA (Supplementary information, Figure S2A).
Precise labeling of telomeres with thioredoxin-fused TALEs (TTALEs). (A) Schematic illustration of TALEs fused with various solubility-enhancing peptides (X) to label telomeres. (B) Co-localization analysis of telomeric FISH (red) and Flag-TALEtelo (green) fused with the indicated peptides in HeLa cells. Engineered TALEtelo was visualized by immunostaining with anti-Flag antibody. Representative images using various fusion partners show that precise co-localization with telomeric FISH signals was obtained only using the thioredoxin-fused TALEtelo (TTALEtelo). Dashed lines indicate the nuclear boundary. Scale bars, 5 μm. (C) Histograms showing numbers of telomeric FISH- and peptide-fused TALEtelo(19 bp)-positive dots in nuclei of HeLa cells. n = 50 nuclei per group.
Precise labeling of centromeres with TTALEs. (A) Schematic illustration of TALEs fused with various solubility-enhancing peptides (X) to label centromeres. (B) Co-localization analysis of centromeric FISH (red) and Flag-TALEcentro (green) fused with the indicated peptides in HeLa cells. Engineered TALEcentro was visualized by immunostaining with anti-Flag antibody. Representative images show precise co-localization of centromeric FISH (red) and TRX-fused TALEcentro (TTALEcentro) signals. Dashed lines indicate the nuclear boundary. Scale bars, 5 μm. (C) Histograms showing numbers of centromeric FISH- and peptide-fused TALEcentro(19 bp)-positive dots in nuclei of HeLa cells. n = 50 nuclei per group.
TRX is a small oxidoreductase whose chaperone-like activity has been used to improve the cellular solubility of its fusion partners30,31. We thus investigated whether the redox-catalytic activity of TRX is involved in the precise recognition exhibited by TTALEs. We generated a redox-inactivated TRX mutant by replacing cysteines 32 and 35 with serines32,33,34. Although mutant TTALEtelo and TTALEcentro were expressed at similar levels to their wild-type (WT) counterparts (Supplementary information, Figure S2B), they failed to specifically label telomeric and centromeric loci, respectively (Supplementary information, Figure S2C), indicating that the redox activity of TRX is required for labeling genomic loci. In addition, we investigated whether the precise genomic labeling of TTALEs indirectly relies on the change of nuclear reductive status induced by overexpression of TRX. To this end, HeLa cells were co-transfected with unmodified TALEtelo or TALEcentro together with GAL4-TRX, a nuclear-localized TRX33,35,36 (Supplementary information, Figure S2D). Co-expression of conventional TALEs with non-fused TRX failed to target TALEs to their corresponding genomic loci (Supplementary information, Figure S2E-S2G). We treated the cells with N-acetyl-cysteine (NAC), a strong antioxidant that increases cellular reductive levels37, and found that suppression of oxidative stress by NAC did not result in correct genomic targeting by conventional TALEs (Supplementary information, Figure S2H). Finally, we compared the effect of TRX with another small redox protein, glutaredoxin (GRX)38,39. The GRX-fused TALEtelo did not exhibit telomere-specific distribution (Supplementary information, Figure S2I). Therefore, our results support a specific chaperone role of TRX when fused with TALE.
Comparison of TTALE with the dCas9/sgRNA system for imaging specific genomic loci
Given that dCas9/sgRNA has also been used as a tool to visualize specific genomic loci15, we next compared the imaging quality of TTALE with that of dCas9/sgRNA in HeLa cells (Supplementary information, Figure S3A). EGFP-TTALEtelo exhibited clearer and sharper fluorescence signals at telomeric loci compared with dCas9/sgRNA, with significantly lower background in the nucleoplasm (Supplementary information, Figure S3B and S3C). After normalization for background noise, the specific fluorescence intensity of EGFP-TTALEtelo was 4.21-fold higher than that of dCas9/sgRNA (Supplementary information, Figure S3D). In addition, flow cytometry showed a much higher transfection efficiency using EGFP-TTALEtelo compared with EGFP-dCas9/sgRNA in multiple cell types tested (Supplementary information, Figure S3E and S3F).
Imaging chromatin dynamics during mitosis with TTALEs
To visualize the dynamic distribution of telomeres and centromeres, we imaged HeLa cells at different mitotic phases using TTALEtelo and TTALEcentro probes. As in interphase cells, we observed nearly perfect overlap between TTALEtelo and telomeric FISH signals and between TTALEcentro and centromeric FISH signals during prophase, metaphase, anaphase, and telophase (Supplementary information, Figure S4A and S4B). In addition, telomeres and centromeres could be simultaneously visualized at all stages of mitosis by co-expression of mCherry-TTALEtelo and EGFP-TTALEcentro(Figure 4A and Supplementary information, Movie S1). These data indicate that binding of TTALEtelo and TTALEcentro probes to telomeric and centromeric DNAs, respectively, is stable and specific throughout mitosis, and further suggest that the expression of TTALEs has minimal effect on mitosis in HeLa cells.
TTALE-mediated imaging of telomeres and centromeres in mitotic HeLa cells, hESCs, and the isogenic cell derivatives of hESCs. (A) Dynamic distribution of telomeres and centromeres at different stages of mitosis. Live HeLa cells co-expressing EGFP-TTALEcentro and mCherry-TTALEtelo were imaged at different mitotic phases. Scale bars, 5 μm. (B) Schematic diagram showing derivation of isogenic cell types from hESCs. hMSCs, hNSCs, and hVSMCs were differentiated from hESCs, and hNSCs were further differentiated into postmitotic neurons or reprogrammed into iPSCs. (C-D) Structured illumination microscopy (SIM) images showing co-localization of centromeric FISH (red) and Flag-TTALEcentro (green) (C) or telomeric FISH (red) and Flag-TTALEtelo (green) (D) signals in the indicated cell types. The number of each scatter plot represents the Pearson r value to show the linear correlation between FISH and TTALEtelo or TTALEcentro signals. Dashed lines indicate the nuclear boundary. Scale bars, 5 μm.
TTALE-based imaging in human stem cells, differentiated cells, and oocytes
We differentiated human embryonic stem cells (ESCs) into two types of adult stem cells, neural stem cells (NSCs) and mesenchymal stem cells (MSCs), and two terminally differentiated cell types, vascular smooth muscle cells (VSMCs) and postmitotic neurons7,8,37,40,41,42,43 (Figure 4B and Supplementary information, Figure S5A-S5E). ESC-derived NSCs were also reprogrammed to generate induced pluripotent stem cells (iPSCs; Supplementary information, Figure S5F-S5G). All ESCs and their isogenic derivatives, the iPSCs, and five transformed human cell lines (HEK293, U2OS, HeLa, HepG2, and MCF7) were employed to evaluate imaging efficiency using EGFP-TTALEs. In all of these cell types, TTALEtelo and TTALEcentro signals perfectly co-localized with telomeric and centromeric FISH signals, respectively (Figure 4C and 4D, Supplementary information, Figure S4C and S4D), indicating that TTALEs are amenable to accurate and high-quality imaging for all human cells tested. Importantly, we also tested the performance of TTALE imaging using unfertilized human oocytes44. We microinjected EGFP-tagged TTALEtelo or TTALEcentro constructs directly into human oocytes and performed live cell imaging immediately after microinjection for 8-12 h. As in cultured cell lines, telomeric and centromeric signals were clearly visible (Supplementary information, Figure S4E, S4F and Movie S2).
TTALE-based imaging of rDNA and single-gene loci
In the human genome, nucleolar organizer region (NOR)-related ribosomal DNAs (NOR-rDNAs) are composed of up to 400 repetitive DNA sequences45. Human 45S rDNA loci encoding 18S, 5.8S, and 28S rRNAs are organized as clusters within NORs. To visualize and monitor NOR-rDNAs, we generated a TTALE targeting a 19-bp DNA sequence (5′-TACCCTACTGATGATGTGT-3′) from 28S rDNA repeats (TTALErDNA; Figure 5A). In MSCs, TTALErDNA exhibited a punctate staining pattern predominantly around nucleoli, whereas a conventional TALE targeting the same sequence labeled cytosolic aggregates (Supplementary information, Figure S6A and S6B). Signals from 3D-FISH probes completely overlapped with TTALErDNA signals at the endogenous 28S rDNA loci (Supplementary information, Figure S6D). Combining mCherry-TTALErDNA, immunostaining with EGFP-Rev (a nucleolus-specific HIV protein46), nucleolin, and fibrillarin, and structured illumination microscopy-transmission electron microscopy (SIM-TEM) imaging with ultrahigh resolution, we further observed that the 28S rDNA loci were predominantly localized along the surface of the nucleolus or occasionally outside the nucleolus similar to pseudo-NORs47,48, and were rarely found inside the nucleolus (Figures 5B, Supplementary information, Figure S6C, S6D, and S6E). We further validated the TTALErDNA-mediated high-quality imaging in diverse human cell lines (Figure 5C) and human oocytes (Supplementary information, Figure S6F), and at different mitotic phases in HeLa cells (Figure 5D). To simultaneously visualize telomeres, centromeres, and 28S rDNA sequences, hMSCs were co-transfected with mCherry-TTALEtelo, YFP-TTALEcentro, and CFP-TTALErDNA. Our results demonstrate that the TTALE system is compatible with multiplex imaging of different genomic loci at the single-cell level (Supplementary information, Figure S6G). In addition to non-gene loci, we further demonstrated that TTALEs can be used for gene-coding genomic loci using MUC4 as an example in interphase hMSCs and at different mitotic phases in HeLa cells (Supplementary information, Figure S6H-S6K)15,16,49,50,51,52.
TTALE-based imaging of NOR-rDNAs. (A) Schematic diagram showing distribution and structural features of NOR-rDNAs in the human genome. (B) Co-localization analysis of mCherry-TTALErDNA (red) and EGFP-Rev (green, labeling nucleolus) signals captured by SIM-TEM. Arrowhead indicates mCherry-TTALErDNA signals at perinucleolar regions. Arrow indicates the fiducial marker (blue) for precise alignment of SIM and TEM images. Scale bars, 2 μm. (C) Live cell co-localization analysis of mCherry-TTALErDNA (red) and EGFP-Rev (green) in the indicated cell types. Dashed lines indicate the nuclear boundary. Scale bars, 5 μm. (D) Visualization of rDNA at different stages of mitosis in HeLa cells using mCherry-TTALErDNA (red). Hoechst was used to stain DNA (blue). Scale bars, 5 μm.
TTALE-based imaging to track genomic changes during human aging
Human cellular aging is driven by both genomic and epigenomic alterations3,7,8,37,40,42,43,53. We next applied TTALE imaging to monitor genomic changes during human cellular aging. Telomere attrition is an established hallmark of aging3,54. We used TTALEteloto monitor changes in telomere length in three established human aging systems: (1) Werner syndrome (WS) MSCs derived from WRN-deficient ESCs7,55,56 (Figure 6A and 6B, and Supplementary information, Figure S7A), (2) MSCs differentiated from iPSCs derived from Hutchinson-Gliford progeria syndrome (HGPS) patients (HGPS-GC-MSCs as an isogenic control line differentiated from LMNA gene-corrected HGPS-iPSCs)40,43,57 (Figure 6A and 6B, and Supplementary information, Figure S7B), and (3) hMSCs undergoing replicative senescence in culture (Figure 6A and 6B)58. Each aging model showed a substantial reduction in telomere length determined by genomic quantitative PCR (Supplementary information, Figure S7C) as well as a decrease in TTALEtelosignal using SIM imaging (Figure 6C). We also demonstrated in a flow cytometry-based assay a marked decrease of fluorescence intensity of mCherry-TTALEtelo in WS-MSCs, relative to WT-MSCs, using co-expressed NLS-EGFP as a transfection control (Supplementary information, Figure S7D-S7E). In addition, we examined whether MSC aging is associated with the altered epigenetic status of centromeric DNA using EGFP-TTALEcentro. TTALEcentro signals were more diffused and less intense in WS-specific human MSCs compared with their WT counterparts (Figure 6D-6F and Supplementary information, Movies S3 and S4). This is consistent with the reported heterochromatin disorganization at centromeres in WS MSCs7, which leads to active transcription from the disorganized centromeric repetitive elements (Supplementary information, Figure S7F). In WRN-deficient ESCs and NSCs used as negative controls, the telomeric length, condensation state of centromeres, and transcription from centromeric elements were not altered by WRN deficiency (Supplementary information, Figure S8A-S8F).
TTALE-mediated imaging of telomeres and centromeres during human stem cell aging. (A) Schematic diagram of three established human stem cell aging models. (B) SIM images showing EGFP-TTALEtelo-labeled telomeres in WS-MSCs vs WT-MSCs at passage 6 (top), HGPS-MSCs vs HGPS-GC-MSCs at passage 8 (middle), and LP-WT-MSCs at passage 12 vs EP-WT-MSCs at passage 6 (bottom). Magnified images of the boxed regions are in the lower-left corner. Dashed lines indicate the nuclear boundary. Scale bars, 5 μm (wide-field images) or 10 μm (magnified images). (C) Decrease of EGFP-TTALEtelo fluorescence intensity with hMSC aging. The scatter plot displays the fluorescence intensity of EGFP-TTALEtelo-labeled telomere puncta normalized by the average fluorescence intensity of the nucleus in the indicated cells. n = 50 nuclei per group; ***P < 0.001. (D) SIM images of centromeres labeled by EGFP-TTALEcentro in WS-MSCs and WT-MSCs. Bottom panels show magnified images of boxed regions from the top panels. Dashed lines indicate the nuclear boundary. Scale bars, 5 μm. (E) Intensity profiles of TTALEcentro signals across the dotted lines (12 μm in length) in the bottom panels of D. (F) Histogram showing the percentage of fluorescence-labeled area relative to nuclear area in the indicated cells. n = 50 nuclei per group; ***P < 0.001.
We also investigated potential changes of NOR-rDNA loci in aged human stem cells. The fluorescence intensity of mCherry-TTALErDNA was significantly lower in the nuclei of senescent WS-MSCs compared with WT-MSCs (Figure 7A-7C); a co-transfected nuclear-targeted GFP (NLS-GFP) was used as an internal control. Furthermore, flow FISH and PCR indicated that the copy number of NOR-rDNA repeats was significantly decreased in senescent WS-MSCs (Figure 7D and 7E), whereas no significant decrease was observed when the copy number of GAPDH locus was analyzed (Supplementary information, Figure S9A). Similar reductions of NOR-rDNA copy number and mCherry-TTALErDNA fluorescence intensity were also observed in HGPS-MSCs undergoing accelerated senescence and in replicative senescent MSCs (Supplementary information, Figure S9B-S9G). To identify whether the NOR-rDNA attrition observed during premature human MSC senescence can be extended to human physiological aging, peripheral bloods from young (6-10 years old) and old (69-72 years old) donors were obtained to detect the NOR-rDNA copy number, as well as telomere length. As expected, we observed significant shortening of telomeres in blood samples from old individuals (Figure 7F, left panel). Importantly, the copy numbers of NOR-rDNA in peripheral blood of old donors were also diminished relative to those of young donors (Figure 7F, right panel). Together, using TTALE-based imaging, we have not only validated telomere attrition and centromere disorganization in senescent human cells7,40,43, but also provided the strong evidence that human aging is associated with attrition of NOR-rDNA repeats.
TTALE-based imaging indicating physical attrition of NOR-rDNAs in senescent WS-MSCs. (A) SIM images showing mCherry-TTALErDNA-labeled NOR-rDNA in WS-MSCs and WT-MSCs. NLS-EGFP was co-expressed to monitor transfection efficiency. Dashed lines indicate the nuclear boundary. Scale bars, 5 μm. (B) Intensity profiles of TTALErDNA signals across the solid lines (10 μm in length) in A. (C) Histogram showing fluorescence intensity of mCherry-TTALErDNA normalized by that of NLS-GFP in A. n = 50 nuclei; ***P < 0.001. (D) Quantitative FISH (FACS) analysis of NOR-rDNA in WS-MSCs and WT-MSCs. Left panel: primary result of FACS. Right panel: histogram showing lower average intensity of NOR-rDNA FISH signal in WS-MSCs compared with WT-MSCs. Data are presented as mean ± SEM; n = 3; ***P < 0.001. (E) Quantitative PCR analysis showing diminished rDNA copy numbers in WS-MSCs relative to WT-MSCs. Data are presented as mean ± SEM; n = 3; **P < 0.01. (F) qPCR analysis of of telomere length (left) and NOR-rDNA copy number (right) in the genomic DNA of peripheral blood samples collected from young and old donors. n = 8 (young donors) or 9 (old donors); ***P < 0.001; *P < 0.05.
We next investigated whether fluorescence-tagged TTALEs can be used for studying aging-associated DNA damage responses (DDRs) at telomeric and centromeric loci. WT- and WRN-deficient MSCs were transfected with mCherry-TTALEtelo or mCherry-TTALEcentro and aging-associated DDR was determined by co-localization of anti-γ-H2AX immunostaining with telomeres or centromeres. We observed significantly increased γ-H2AX signals at both telomeres and centromeres in WRN-deficient MSCs relative to WT controls (Supplementary information, Figure S10A-S10D). As a positive control, activation of DDR by treatment of WT MSCs with the DNA damage agent hydroxyurea59 induced a similar accumulation of γ-H2AX signals at these genomic repetitive elements (Supplementary information, Figure S10A-S10D). Collectively, these results establish TTALEs as a robust tool for the study of human aging-related biology.
TTALE-based in vivo imaging to track telomere attrition in telomerase-deficient mice
Next, we investigated whether the TTALE system can be employed to directly investigate organ and tissue aging in vivo in an accelerated-aging mouse model. For this purpose and as a proof of concept, we utilized previously reported telomerase-deficient mice (G3 mTerc−/−) which showed significant telomere attrition compared with WT mTerc+/+ mice60,61,62,63 (Figure 8A and Supplementary information, Figure S11A-S11C). We developed a lentivirus-based EGFP-TTALEtelo system (Supplementary information, Figure S11D and S11E and Data S1), and verified its utility as a tool to precisely label telomeres in cultured human U2OS cells and mouse OP9 cells with very high efficiency (transduction efficiency is over 95%) (Supplementary information, Figure S11F-S11G). Next we delivered the purified lentiviruses directly into the hippocampus, liver, and anterior tibial muscle of WT and G3 mTerc−/− mice (Figure 8A). In each of these tissues, we observed that both telomere length (Supplementary information, Figure S11C) and EGFP-TTALEtelo signals (Figure 8B-8E) were markedly reduced in G3 mTerc−/− mice compared with WT mice. These results demonstrate that TTALEs can be used as a simple tool for studying tissue and organ aging in vivo at the single-cell level.
Lentiviral TTALE-mediated in situ telomere imaging in mouse tissues. (A) Schematic diagram of TTALE-based telomere imaging in mouse liver, anterior tibial muscle, and hippocampus. (B-D) Lentiviral EGFP-TTALEtelo-mediated telomere imaging in anterior tibial muscle (B), liver (C), and hippocampus (D) in WT mTerc+/+ and G3 mTerc−/− mice. Dye for wheat germ agglutinin (WGA), antibody against human serum albumin (anti-ALB), and antibody against NeuN (anti-NeuN) were used to label (in red) the outline of anterior tibial muscle cells (B), the cytoplasm of liver cells (C), and nuclei of the hippocampus (D), respectively. Magnified images of the boxed region are shown in the lower panels. Dashed lines indicate the nuclear boundary. Scale bars, 50 μm (wide-field images) or 2 μm (magnified images). (E) Normalized fluorescence intensities of EGFP-TTALEtelosignals in B-D. n = 100 nuclei; ***P < 0.001.