Abstract
Glioblastoma (GBM) is associated with poor prognosis despite aggressive surgical resection, chemotherapy, and radiation therapy. Unfortunately, this standard therapy does not target glioma cancer stem cells (GCSCs), a subpopulation of GBM cells that can give rise to recurrent tumors. GBMs express human cytomegalovirus (HCMV) proteins, and previously we found that the level of expression of HCMV immediate-early (IE) protein in GBMs is a prognostic factor for poor patient survival. In this study, we investigated the relation between HCMV infection of GBM cells and the presence of GCSCs. Primary GBMs were characterized by their expression of HCMV-IE and GCSCs marker CD133 and by patient survival. The extent to which HCMV infection of primary GBM cells induced a GCSC phenotype was evaluated in vitro. In primary GBMs, a large fraction of CD133-positive cells expressed HCMV-IE, and higher co-expression of these two proteins predicted poor patient survival. Infection of GBM cells with HCMV led to upregulation of CD133 and other GSCS markers (Notch1, Sox2, Oct4, Nestin). HCMV infection also promoted the growth of GBM cells as neurospheres, a behavior typically displayed by GCSCs, and this phenotype was prevented by either chemical inhibition of the Notch1 pathway or by treatment with the anti-viral drug ganciclovir. GBM cells that maintained expression of HCMV-IE failed to differentiate into neuronal or astrocytic phenotypes. Our findings imply that HCMV infection induces phenotypic plasticity of GBM cells to promote GCSC features and may thereby increase the aggressiveness of this tumor.
Similar content being viewed by others
Main
GBM is the most prevalent and the most aggressive primary malignancy of the central nervous system in adults. It is a highly vascularized and infiltrating tumor, rarely cured and prone to recurrence. The median duration of survival after diagnosis is less than 15 months, despite aggressive therapy consisting of surgical resection and concomitant radiotherapy and chemotherapy.1 Surgical resection of GBMs is typically incomplete, as they are located in the brain and are highly infiltrative. Postoperative radiotherapy and chemotherapy fail to eradicate all remaining GBM cells. Thus, a breakthrough in identifying a new treatment option leading to a cure of this disease is still lacking.
GBMs contain a subpopulation of highly tumorigenic cells with unlimited capacity for self-renewal that are commonly resistant to standard therapy. Phenotypically and functionally, these cells resemble neural stem cells and, when implanted in immunodeficient mice, can generate new tumors. As a result, they are referred to as glioma cancer initiating cells or glioma cancer stem cells (GCSCs) (reviewed in Lima et al.2). Because of their apparent pivotal role in gliomagenesis and tumor recurrence after therapy, GCSCs are a major focus of research whose ultimate goal is to identify more effective therapies for GBM patients.
GCSCs were first identified by their surface expression of CD133, based on the findings that these cells grow as neurospheres under nonadherent conditions and that tumors form in vivo after implantation of only 100 CD133-positive GBM cells but not after implantation of 105 CD133-negative GBM cells.3 The importance of CD133 as a marker of tumor aggressiveness was corroborated by the correlation between CD133 expression in brain tumors and a poor clinical prognosis.4, 5, 6 However, later studies revealed that CD133-negative cells can give rise to CD133-positive cells7, 8, 9 and that both CD133-positive and CD133-negative GBM cells can initiate the development of highly aggressive tumors.10 Moreover, diverse GCSC types – all capable of self-renewal and tumor initiation – coexist within the same GBM.10 These cells often express markers associated with stem cells such as Sox2, Notch, and Oct-4.11, 12, 13 This intratumoral heterogeneity and the resulting aggressiveness of GBMs are influenced by the location of the tumor within the brain and by tumor-associated microenvironmental factors (reviewed in Stopschinski et al.14). While the general validity of CD133 as a major GCSC marker is still debated and its exact function in gliomagenesis remains poorly understood, other GCSC markers have been identified, including Notch1, Oct4, Sox2, and Nestin.4, 14, 15 The high levels of expression of these markers appear to functionally induce or maintain features that are characteristic of GCSCs.
We have focused on develo** and testing novel treatments for GBM based on two observations: that 99% of GBMs contain human cytomegalovirus (HCMV) proteins16, 17 and nucleotide sequences, and that the degree of HCMV protein expression in GBMs is a prognostic factor for patient survival.18
HCMV is a herpesvirus that infects 70–100% of the world’s population. After an active primary infection, usually asymptomatic or subclinical in immunocompetent individuals, the virus establishes latency in the bone marrow and peripheral blood. Latent infections can be reactivated by inflammation. In immunocompromised individuals, primary HCMV infection and reactivation are significantly associated with morbidity and mortality.19, 20
In a clinical trial, we found that the antiviral drug valganciclovir as an add-on to standard therapy led to high survival rates among GBM patients. In a retrospective analysis of patients continuously receiving such therapy for more than 6 months, the 2-year survival rate was 90% and median overall survival was 56.4 months, as compared with 18% and 13.5 months, respectively, in contemporary controls.17 These results suggest that HCMV has an oncogenic or an oncomodulatory role in GBMs, and highlight the possibility that valganciclovir may eliminate or modulate the behavior of GCSCs that may not be targeted with conventional therapies.
In light of these findings, we hypothesized that HCMV infection of GBM cells and the maintenance of a GCSC phenotype could be interrelated events. To test this hypothesis, here we investigated potential co-expression of a GCSC marker with HCMV immediate-early protein in a series of human clinical GBM specimens, and experimentally assessed the ability of HCMV infection to induce a GCSC phenotype in primary human GBM cells.
Results
CD133 and HCMV-IE are co-expressed in GBM cells and are both predictive factors for patient survival
We first analyzed, by flow cytometry, the expression of CD133 and HCMV immediate early protein (HCMV-IE) in GBM cells immediately after dissociation from fresh, surgically excised tumors obtained from 21 patients (Table 1). In 20 (95%) of 21 GBMs 1–52% of cells expressed IE proteins (Figure 1a). In 19 of those 20 tumors, 1–70% of cells expressed CD133. In 16 tumors, 0.7–12% of cells co-expressed CD133 and HCMV-IE proteins at various levels (Figures 1b and c).
CD133 and HCMV-IE as prognostic markers for GBM. (a) Immunohistological staining for HCMV IE. Primary antibody was omitted from the negative control. Staining for muscle cell (SMC) alpha actin served as an isotype control. (b) Expression of CD133 and HCMV-IE in GBM tumor tissues, determined by flow cytometry (the line indicates the median percentage value). (c) Representative dot plots from flow cytometry of cells double-stained for HCMV-IE protein (FITC) and CD133 (PE). (d and e) Kaplan–Meier survival analysis of patients whose GBMs had high versus low expression of CD133 or HCMV-IE (The median values were used as cutoffs to split the patients into groups with high and low levels of protein expression in their tumors. P=0.0034 for CD133, P=0.0063 for HCMV-IE)
We next assessed the extent to which the percentage of IE-positive, CD133-positive, or IE/CD133 double-positive cells could be prognostic markers within this small cohort of GBM patients. We used the median values as cutoffs to split the patients into groups whose tumors had a high or low percentage of CD133 and IE-expressing cells. Kaplan–Meier plots showed that higher percentages of CD133-positive and IE-positive cells were associated with poor patient survival (Figures 1d and e, P=0.0034 for CD133, P=0.0063 for HCMV-IE).
The finding that both CD133 and IE are significant prognostic markers for GBM suggested that their expression was interdependent. To assess this possibility, we plotted the percentage of CD133-positive cells against the percentage of IE-positive cells in each tumor analyzed. Linear regression analysis showed a positive correlation (P=0.0263, Figure 2a). HCMV was expressed in 12–100% of CD133-positive cells from GBM (Figure 2b). Patients with higher extent of HCMV-IE and CD133 co-expression also tended to have overall shorter survival time (P=0.0552, Figure 2c).
Correlation between CD133 and HCMV-IE expression in GBMs. (a) The percentage of CD133-expressing cells was plotted against the percentage of HCMV-IE-expressing cells in each GBM tumor analyzed. Linear regression analysis revealed a positive correlation (R2=0.234, P=0.0263). (b) The percentage of HCMV-IE-positive cells within CD133-positive population of GBM tumor cells, analyzed by flow cytometry (the line represents the median value). (c) Kaplan–Meier survival analysis of patients whose GBMs had high or low CD133/HCMV-IE co-expression (The median value was used as a cutoff to split the patients into two groups, P=0.0552)
The high degree of correlation between IE and CD133 and the high prevalence of IE expression in CD133-positive cells suggest that HCMV infection may induce or maintain a GCSC phenotype (as defined by CD133 expression) in GBM cells. Alternatively, HCMV-infected cancer cells might alter the tumor microenvironment or act in a paracrine manner to support the maintenance of CD133-positive GCSCs.
HCMV infection induces a cancer stem cell phenotype in primary GBM cells
To determine whether HCMV infection of tumor cells induces a cancer stem cell phenotype, we isolated primary GBM cells from fresh surgically excised tumor specimens and cultured them under adherent conditions. Infection of the cells with HCMV resulted in significantly increased expression of CD133 RNA and protein (Figures 3a and b). Infection also upregulated the stem cell marker Notch1 but did not affect the transcription of other stem cell markers (SOX2, Oct-4, or Nestin) (Figures 3c and d). The upregulation of CD133 and Notch1 indicates that HCMV infection could indeed lead to the development or maintenance of a GCSC phenotype.
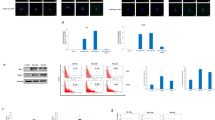
HCMV infection of GBM cells upregulates CD133 and Notch-1 expression. Primary GBM cells were cultured under adherent conditions and infected with HCMV in vitro. Control cells were not infected. (a) Fold change in CD133 transcription in infected cells compared with uninfected cells, assessed 3 and 7 days after infection. **P=0.0027 versus uninfected cells. Values are mean±S.D. of three GBM-derived cell cultures. (b) Percentage of CD133-positive cells determined by flow cytometry 3 and 7 days after HCMV infection. *P=0.028, *P=0.042 versus uninfected cells. Values are mean±S.D. of three GBM-derived cell cultures. (c) Level of transcription of stem cell markers assessed 3 days after HCMV infection. **P=0.0029 versus uninfected cells. Values are mean±S.D. of three GBM-derived cell cultures. (d) Immunofluorescence staining for Notch-1 (Red), HCMV IE (green), and nucleus (DAPI, blue) 3 days after infection in GBM cells
Next, we assessed the ability of HCMV-infected cells to grow under nonadherent conditions and form neurospheres, a behavior typical of GCSCs. Primary GBM cells grown under adherent conditions were infected with HCMV and cultured in stem cell culture medium, which does not support adherent growth. Uninfected GBM cells served as a control. HCMV infection substantially increased the ability of GBM cells to form neurospheres (Figure 4a) and upregulated the expression of CD133, Notch 1, SOX-2, Oct-4, and Nestin (Figures 4f and g). However, it did not significantly affect the expression of BMI1, another GCSC maker (Figure 4g). Thus, HCMV infection of primary adherent GBM cells in vitro induced nonadherent growth of neurospheres that exhibited enhanced expression of five GCSC markers examined. Interestingly, when working with primary cells that had been passaged eight times or more, we occasionally observed neurosphere formation without the need for previous in vitro infection with HCMV in three primary cell cultures. Few cells within these cultures displayed HCMV-IE reactivity in the cytoplasm when neurospheres were formed (Figure 4h). This staining pattern resembles that of HCMV-positive cells in GBM tissues, while cytoplasmic IE reactivity is rarely observed in in vitro infected cells where HCMV-IE is located in the nucleus.
HCMV infection of GBM cells induces neurosphere formation in culture. GBM cells cultured under adherent conditions were infected with HCMV and transferred to stem cell medium. (a) Light microscopic images of HCMV infected and uninfected GBM cell lines 7 and 12 days after transfer of the cells to stem cell medium. Magnification, × 10. The inset shows the neurospheres at higher magnification, × 20. (b) Light microscopic images of infected and uninfected cells treated with GCV or gamma secretase inhibitor (GSI) 7 days after transfer to stem cell medium. Magnification, × 10. (c) Apoptosis of HCMV-infected GBM cells, untreated or treated with GCV, cultured in stem cell medium assessed by AnnexinV-FITC stain. (d) Quantification of cell viability of GBM and MRC5 cells untreated or treated with GCV for 7 days, using the MTS assay. The values are presented as mean±S.D. *P=0.004, **P= 7.8 10−5. (e) Representative light microscopic images (magnification, × 4) of GBM and MRC5 cells untreated or treated with GCV for 7 days. (f) Fold change in CD133 mRNA expression in GBM-derived neurospheres 7 and 12 days after transfer of cells to stem cell medium (n=6). (g) Fold change in mRNA expression of the stem cell markers Notch 1, SOX2, OCT 4, Nestin, and BMI1 in neurospheres 12 days after transfer of cells to stem cell medium (n=3) ***P=0.0001, **P=0.0099, *P=0.0368, *P=0.019 versus uninfected cells The results are presented as mean±S.D. with respect to uninfected cells. (h) Representative image of cultured GBM cells at passage 8, not infected in vitro, immunostained for HCMV-IE (brown stain) and stained with DAPI (blue nuclei)
Ganciclovir treatment is toxic to uninfected and HCMV-infected GCSCs
To confirm that neurosphere formation was due to HCMV infection, and to assess the effect of antiviral therapy on the development of this cancer stem cell phenotype, we treated the cells with ganciclovir (GCV). This drug inhibits the viral DNA polymerase and is used clinically to treat acute HCMV infection. GCV inhibited neurosphere formation and was highly toxic to both infected and uninfected GBM cells (Figures 4b and c) cultured under stem cell conditions. Moreover, this cytotoxic effect preferentially affected uninfected GBM cells compared when uninfected normal MRC5 fibroblasts in culture (Figures 4d and e).
A gamma secretase inhibitor blocks neurosphere formation of GCSC
The Notch pathway is important in the maintenance of GCSCs. As HCMV infection of GBM cells upregulated Notch1, we determined whether this pathway mediates the formation of neurospheres upon HCMV infection. Indeed, treatment with gamma secretase inhibitor (GSI), which inhibits the Notch pathway, prevented neurosphere formation (Figure 4b).
HCMV infection prevents differentiation of cancer stem cells
To investigate the ability of HCMV-induced neurospheres to differentiate, we cultured them in cell culture medium that triggers differentiation of stem cells into astrocytes and neurons (Figure 5a). Remarkably, HCMV-infected GBM cells remained mostly refractory to such differentiation-inducing conditions, and the majority of infected cells did not express either neuronal or astrocytic markers (Figures 5b and c). These results suggested that HCMV holds GBM cells in an undifferentiated state.
HCMV infection blocks GBM stem cell differentiation. Cells from neurospheres were cultured under conditions that drive differentiation into neurons and astrocytes. (a) Light microscopic images of GCSC spheres cultured under differentiation conditions at 3 and 7 days (n=2) (magnification, × 10). (b) Immunofluorescence staining of the differentiated spheres for the neuronal marker Tuj1 (green), the astrocytic marker GFAP (green), HCMV-IE (red), and nucleus (DAPI, blue). (c) Percentage of cells expressing Tuj1 or GFAP within the subpopulations of cells expressing or not expressing HCMV-IE. The values are presented as mean+S.D. *P=0.0005, **P= 2.5 10−6
Discussion
In this study, we found that the level of expression of HCMV-IE protein in freshly isolated GBM cells correlates with the level of expression of CD133, a putative marker of GCSCs. We also found that in vitro HCMV infection of primary GBM cells induces a stem cell phenotype, as determined by an increased expression of the GCSC markers CD133, Notch1, SOX2, Oct4, and Nestin, and by the acquired ability of GBM cells to grow as neurospheres under nonadherent conditions. HCMV infection also appeared to hold cells derived from neurospheres in an undifferentiated state – under culture conditions that promote their differentiation into astrocytic or neural lineages. Thus, HCMV infection of GBM cells can increase the number of GCSCs and maintain them in an undifferentiated stem-like state and thereby possibly increase the aggressiveness of GBM tumors.
Although we have consistently reported the expression of HCMV proteins and nucleic acid sequences in primary tumors, the level of endogenous expression of HCMV in primary GBM cell cultures was very low to undetectable. It is possible that the cells that are amenable to ex vivo culture under adherent conditions are less likely to harbor HCMV. Alternatively, it is possible that HCMV modulates its expression profile in a cell culture setting. In agreement with this hypothesis, we have observed fluctuating levels of HCMV-IE in cultured GBM cells. Also, after several passages (at least 8), we have occasionally observed spontaneous growth of these cells as neurospheres, without the need of in vitro infection. These cultures were then positive for HCMV-IE proteins in the cytoplasm (only in few cells). Thus, the fluctuation in the expression of HCMV-IE and the ability of cells to form neurospheres appeared to be interrelated.
GBMs are highly heterogeneous tumors featuring pronounced endogenous stress and rampant genomic instability.21, 22 Four cellular phenotypes have been identified: proneural, neural, mesenchymal, and classical.23 Each phenotype has a slightly different prognosis, with the mesenchymal subtype being the most aggressive and leading to the shortest life expectancy. As shown by single-cell sequencing, all four cellular subtypes are present in each GBM, further emphasizing the complexity of this tumor type.24 Although GCSCs are present at a low frequency, they can give rise to tumors with characteristics similar to those of the primary tumor they are derived from. GCSCs have been described as tumor cells that display stem cell properties, including unlimited self-renewal, tumorigenic potential, asymmetric cell division, and multipotency – the ability to differentiate into both rapidly proliferating progenitor-like cells and more differentiated GBM cells.2 As these cells are resistant to standard therapy, they are considered a major source of GBM recurrence, which makes them a promising target for novel, more effective therapies.
The mechanisms that maintain GCSC in an undifferentiated state are highly complex and remain to be fully elucidated. Nevertheless, several pathways involving GCSC markers appear to have a major role. CD133 expression appears essential, as silencing of this protein in GBM neurospheres impairs their self-renewal and their ability to give rise to tumors in vivo.25 The Notch pathway is essential for cell-cell communication and can maintain GCSCs in an immature state by promoting their self-renewal and inhibiting their differentiation.12 Nestin overexpression increases the growth rate and migration of glioma cells and their adhesion to the extracellular matrix.11 Both SOX2 and Oct4 participate in the reprogramming of differentiated cells into induced pluripotent stem cells and are overexpressed in various types of cancer.13
HCMV infection of GBM cells upregulated all of the above-mentioned markers when cells were cultured in stem cell conditions, highlighting a potential role for HCMV in the induction of GCSCs. Moreover, GBM cells grown as neurospheres and expressing HCMV-IE were unable to differentiate into astrocytic or neuronal phenotypes, highlighting a potential role for HCMV in the maintenance of GCSC state. These findings are in line with our previous results showing that HCMV infection of fetal neural progenitor inhibits their differentiation.26 Other studies have suggested that halted differentiation of neuroprogenitor cells is an early step in gliomagenesis.27 Since HCMV proteins and nucleotide sequences seem to be present in most GBMs16, 28, 29, Sphere formation and differentiation Adherent GBM primary cells were infected with HCMV and cultured under adherent conditions. Three days after infection, the cells were dissociated with 0.5% trypsin-EDTA and transferred to a new flask containing stem cell medium (as described above). Alternatively, GBM primary cells that were not infected in vitro were transferred to stem cell medium to assess the possibility of non-adherent growth as neurospheres. Twelve days later, spheres or cells dissociated from spheres were cultured in NeuroCult NS-A Differentiation Kit (Stemcell Technologies, Vancouver, BC, Canada) to induce differentiation of GCSCs to astrocytes or neurons. Patient survival was analyzed with Kaplan–Meier curves. Median values were used as cutoff values to define two groups of patients, which were compared by log-rank test. Differences in protein or transcript expression in infected and uninfected cells were analyzed by Student's t-test. In studies of cultured cells, the results are representative of at least three independent experiments with cells from GBMs of three different patients. The statistical analysis was conducted using the GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA) or Microsoft Excel version 14.0.7147.5001 (Microsoft, Redmond, WA, USA).Statistics
Abbreviations
- GBM:
-
glioblastoma
- GCSC:
-
glioma cancer stem cell
- HCMV:
-
human cytomegalovirus
- IE:
-
HCMV immediate early protein
- GCV:
-
ganciclovir
- GSI:
-
gamma secretase inhibitor
- GFAP:
-
glial fibrillary acidic protein
References
Wen PY, Kesari S . Malignant gliomas in adults. N Engl J Med 2008; 359: 492–507.
Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 2012; 1826: 338–349.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Cheng JX, Liu BL, Zhang X . How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat Rev 2009; 35: 403–408.
Ardebili SY, Zajc I, Gole B, Campos B, Herold-Mende C, Drmota S et al. CD133/prominin1 is prognostic for GBM patient's survival, but inversely correlated with cysteine cathepsins' expression in glioblastoma derived spheroids. Radiol Oncol 2011; 45: 102–115.
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 2008; 14: 123–129.
Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007; 67: 4010–4015.
Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA et al. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery 2008; 62: 505–514; discussion 514-505.
Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer 2008; 122: 761–768.
Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell 2010; 17: 362–375.
Ishiwata T, Teduka K, Yamamoto T, Kawahara K, Matsuda Y, Naito Z . Neuroepithelial stem cell marker nestin regulates the migration, invasion and growth of human gliomas. Oncol Rep 2011; 26: 91–99.
Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B et al. Targeting Notch to target cancer stem cells. Clin Cancer Res 2010; 16: 3141–3152.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676.
Stopschinski BE, Beier CP, Beier D . Glioblastoma cancer stem cells—from concept to clinical application. Cancer Lett 2013; 338: 32–40.
Florio T, Barbieri F . The status of the art of human malignant glioma management: the promising role of targeting tumor-initiating cells. Drug Disc Today 2012; 17: 1103–1110.
Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 2002; 62: 3347–3350.
Soderberg-Naucler C, Rahbar A, Stragliotto G . Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med 2013; 369: 985–986.
Rahbar A, Orrego A, Peredo I, Dzabic M, Wolmer-Solberg N, Straat K et al. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J Clin Virol 2013; 57: 36–42.
Sinclair J . Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J Clin Virol 2008; 41: 180–185.
Soderberg-Naucler C, Fish KN, Nelson JA . Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 1997; 91: 119–126.
Perez-Garcia A, Carrion-Navarro J, Bosch-Fortea M, Lazaro-Ibanez E, Prat-Acin R, Ayuso-Sacido A . Genomic instability of surgical sample and cancer-initiating cell lines from human glioblastoma. Front Biosci 2012; 17: 1469–1479.
Bartkova J, Hamerlik P, Stockhausen MT, Ehrmann J, Hlobilkova A, Laursen H et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene 2010; 29: 5095–5102.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110.
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014; 344: 1396–1401.
Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G, Pelicci G . CD133 is essential for glioblastoma stem cell maintenance. Stem Cell 2013; 31: 857–869.
Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, Soderberg-Naucler C . Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol 2006; 80: 8929–8939.
Hu J, Ho AL, Yuan L, Hu B, Hua S, Hwang SS et al. From the Cover: Neutralization of terminal differentiation in gliomagenesis. Proc Natl Acad Sci USA 2013; 110: 14520–14527.
Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF . Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J Virol 2012; 86: 854–864.
Bhattacharjee B, Renzette N, Kowalik TF . Genetic analysis of cytomegalovirus in malignant gliomas. J Virol 2012; 86: 6815–6824.
Mitchell DA, **e W, Schmittling R, Learn C, Friedman A, McLendon RE et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-oncol 2008; 10: 10–18.
Hadaczek P, Ozawa T, Soroceanu L, Yoshida Y, Matlaf L, Singer E et al. Cidofovir: a novel antitumor agent for glioblastoma. Clin Cancer Res 2013; 19: 6473–6483.
Stern-Ginossar N, Weisburd B, Michalski A, Le VT, Hein MY, Huang SX et al. Decoding human cytomegalovirus. Science 2012; 338: 1088–1093.
Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cell 2010; 28: 5–16.
Venere M, Hamerlik P, Wu Q, Rasmussen RD, Song LA, Vasanji A et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ 2014; 21: 258–269.
Acknowledgements
This work was supported by BILTEMA Foundation, Stichting af Jochnicks Foundation, Sten A Olssons Foundation for Research and Culture, Familjen Erling-Perssons Stiftelse, nexttobe, RATOS, Torsten and Ragnar Söderbergs Foundations, Dan och Jane Olssons Foundation, Swedish Cancer Foundation, Swedish Medical Research Council, Swedish Society for Medical Research (SLS), Goljes Memory Foundation, Magnus Bergvalls Foundation, Swedish Society for Medical Research (SSMF), Inga Britt och Arne Lundbergs Foundation, Danish Cancer Society, Novo Nordisk Foundation, Danish Council for Independent Research, Lundbeck Foundation, Swedish Research Council (K2014-99X-22627-01-4, K2013-57X-12615-16-5) and Tore Nilsons Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Fornara, O., Bartek Jr, J., Rahbar, A. et al. Cytomegalovirus infection induces a stem cell phenotype in human primary glioblastoma cells: prognostic significance and biological impact. Cell Death Differ 23, 261–269 (2016). https://doi.org/10.1038/cdd.2015.91
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2015.91
- Springer Nature Limited