Abstract
Evidence indicates that nitrosative stress and mitochondrial dysfunction participate in the pathogenesis of Alzheimer’s disease (AD). Amyloid beta (Aβ) and peroxynitrite induce mitochondrial fragmentation and neuronal cell death by abnormal activation of dynamin-related protein 1 (DRP1), a large GTPase that regulates mitochondrial fission. The exact mechanisms of mitochondrial fragmentation and DRP1 overactivation in AD remain unknown; however, DRP1 serine 616 (S616) phosphorylation is likely involved. Although it is clear that nitrosative stress caused by peroxynitrite has a role in AD, effective antioxidant therapies are lacking. Cerium oxide nanoparticles, or nanoceria, switch between their Ce3+ and Ce4+ states and are able to scavenge superoxide anions, hydrogen peroxide and peroxynitrite. Therefore, nanoceria might protect against neurodegeneration. Here we report that nanoceria are internalized by neurons and accumulate at the mitochondrial outer membrane and plasma membrane. Furthermore, nanoceria reduce levels of reactive nitrogen species and protein tyrosine nitration in neurons exposed to peroxynitrite. Importantly, nanoceria reduce endogenous peroxynitrite and Aβ-induced mitochondrial fragmentation, DRP1 S616 hyperphosphorylation and neuronal cell death.
Similar content being viewed by others
Main
Nitric oxide (NO) is a neurotransmitter and neuromodulator required for learning and memory.1 NO is generated by NO synthases, a group of enzymes that produce NO from L-arginine. In addition to its normal role in physiology, NO is implicated in pathophysiology. When overproduced, NO combines with superoxide anions (O2·−), byproducts of aerobic metabolism and mitochondrial oxidative phosphorylation, to form peroxynitrite anions (ONOO−) that are highly reactive and neurotoxic. Accumulation of these reactive oxygen species (ROS) and reactive nitrogen species (RNS), known as oxidative and nitrosative stress, respectively, is a common feature of aging, neurodegeneration and Alzheimer’s disease (AD).1
Nitrosative stress caused by peroxynitrite has a critical role in the etiology and pathogenesis of AD.2, 3, 4, 5, 6, 7 Peroxynitrite is implicated in the formation of the two hallmarks of AD, Aβ aggregates and neurofibrillary tangles containing hyperphosphorylated Tau protein.1, 4, 7 In addition, peroxynitrite promotes the nitrotyrosination of presenilin 1, the catalytic subunit of the γ-secretase complex, which shifts production of Aβ to amyloid beta (Aβ)42 and increases the Aβ42/Aβ40 ratio, ultimately resulting in an increased propensity for aggregation and neurotoxicity.5 Furthermore, nitration of Aβ tyrosine 10 enhances its aggregation.6 Peroxynitrite can also modify enzymes, such as triosephosphate isomerase,4 and activate kinases, including Jun amino-terminal kinase and p38 mitogen-activated protein kinase, which enhance neuronal cell death.Immunocytochemistry for 3-NT Neurons were grown on poly-L-lysine coated glass coverslips as previously described38 and fixed with 4% formaldehyde (Ted Pella, Inc.) in PBS for 10 min at RT. Fixed neurons were then permeabilized with 0.1% Triton X-100 in PBS for 5 min. Nonspecific binding was blocked with 3% BSA and 3% FBS in PBS for 1 h at RT. Fixed neurons were then probed with antibodies for 3-NT (1 : 500, Sigma) and an antibody specific for MAP 2 protein (1 : 200, Invitrogen; RT, 2 h), a neuronal marker, followed by conjugated fluorescent secondary antibodies, AlexaFluor594 or AlexaFluor488, respectively, at dilutions of 1 : 500 (RT, 2 h). Chromatin was stained by incubating fixed samples with Hoechst 33342 (1 μg/ml) in PBS at RT for 5 min. To visualize 3-NT using AlexaFluor594, the excitation filter was S555/28 × (Chroma, Bellows Falls, VT, USA) and the emission filter was S617/73m (Chroma). To visualize neurons using AlexaFluor488, the excitation filter was S490/20 × and the emission filter was S528/38m (Chroma). To visualize Hoechst 33342, the excitation filter was S403/12 × and the emission filter was S475/50m. Immunostaining conditions for 3-NT were first optimized along with a blocking control using 10 mM nitrotyrosine to confirm specificity of 3-NT signal. Fluorescence microscopy was performed as previously described.11 Quantification of fluorescence from 3-NT was as follows. Exposure time, brightness and contrast of randomly selected cortical neurons were held constant for all images within same experiment. Using MetaMorph 7.5, a region of interest was selected around each neuron using the MAP 2 label as a guide. This region was transferred to the 3-NT image channel. The fluorescence intensity for each neuron was measured using Show Region Statistics function. Area and intensity/fluorescence data were logged for each neuron. Neurons (25–50) from each treatment were evaluated for a total of over 100 neurons per experiment. Three areas were selected randomly within each image, and the average of their fluorescence intensity was considered as background. The background was subtracted within each image. 3-NT immunofluorescence quantification is expressed as fluorescence per μm2. Neurons were cultured on poly-L-lysine-coated MatTek dishes and pretreated with nanoceria for 3 h. To visualize peroxynitrite in neurons, cell permeable APF (2.4 μM; Life Technologies, Carlsbad, CA, USA) was loaded in Neurobasal medium (phenol red-free) containing 0.2% pluronic acid, 1.8 mM CaCl2, 0.8 mM MgCl2 and Hoechst 33342 (1 μg/ml) for 30 min at 37 °C in a humidified 5% CO2 environment. Excess dye was then removed and replaced with conditioned phenol red-free Neurobasal medium. The APF fluorescent signals were measured in response to SNOC (100 μM) at 2 h. Z-stacks were acquired kee** the exposure time, brightness and contrast constant using excitation S490/20 × and emission S528/38m filters (Chroma). Using MetaMorph 7.5 software (Molecular Devices, Sunnyvale, CA, USA), equal backgrounds were subtracted from each z-stack image (as determined from each experiment’s control images), then z-stack series were summed. Cell soma and processes were selected using a region of interest, as previously described.60 This region was transferred to the APF image channel. The fluorescence intensity for each neuron was measured using Show Region Statistics function. Intensity/fluorescence data were logged for each neuron and data were exported to Excel for further analysis. Nanoceria were synthesized by a wet chemical process as previously described.61 In brief, to prepare nanoceria with a high ratio of Ce3+/Ce4+, Ce (NO3)3 6H2O (5 mM) was dissolved in dH2O and the nitrate precursor was stirred for 15 min then H2O2 (2% v/v) was rapidly added while stirring at 300 r.p.m. The solution was continuously stirred for 1 h to obtain a stable dispersion of CeO2 nanoparticles. Samples were stored at RT. All preparations were sonicated to ensure single nanoparticles (Branson, Danbury, CT, USA) for 45–60 min before use. For cell experiments, nanoceria were diluted in sterile water. Neurons were cultured on poly-L-lysine-coated 35-mm MatTek glass bottom dishes and fixed with 2% paraformaldehyde, 0.15 M sodium cacodylate, pH 7.4, 2.5% glutaraldehyde for 5 min at RT, followed by an additional 30 min on ice. The fixed cells were then washed three times with ice-cold 0.15 M sodium cacodylate and 3 μM calcium chloride for 3 min on ice, followed by post fixation in 1% osmium tetroxide, 0.8% potassium ferrocyanide and 3 μM calcium chloride in 0.15 M sodium cacodylate for 60 min on ice. After washing cells three times with ice-cold ddH2O for 3 min each, the cultures were stained in 2% uranyl acetate for 30 min on ice. Samples were dehydrated with ice-cold 20, 50, 70 and 90% ethanol and then with 100% ethanol at RT. The samples were first infiltrated in 50% ethanol/50% Durcupan ACM (Fluka/Sigma) for 1 h at RT and under agitation, followed by three changes of 100% Durcupan for 3 h. The resin was polymerized at 80 °C for 3–4 days under vacuum. Sectioning was performed using AO/Reichert Ultramicrotome. Ultrathin (80 nm) sections were post stained with uranyl acetate (5 min) and lead salts (2 min) before imaging using a JEOL 1200FX TEM operated at 80 kV. A subset of sections was imaged without post staining. Negatives were shot at a magnification of × 20 000. The negatives were digitized at 1800 d.p.i. using a Nikon CoolScan system, giving an image size of 4033 × 6010 pixels and a pixel resolution of 0.71 nm. The nanoparticle morphology was characterized using high-resolution TEM (HRTEM). The nanoceria preparation was deposited on the carbon-coated copper grid (SPI supplies) for HRTEM analysis. The TEM grid was dipped into the nanoceria preparation by the dip-coating technique. HRTEM micrographs were obtained using FEI Tecnai F30 operated at 300 keV. Nanoceria were isolated by centrifugation at 20 000 r.p.m using Hermle 220.87 fixed angle rotor in Hermle Z-383K Centrifuge (Cole-Parmer, Vernon Hills, IL, USA) for 20 min and the pellets were dried and resuspended in 10 mM sodium phosphate buffer, pH 7.4, 50 μM DTPA. Samples were then transferred onto silicon wafers (Kmbh Associates, Rancho Cordova, CA, USA; CZ Silicon, thickness of wafer: 350 μm) and air dried. The surface chemistry of the nanoparticles was studied using a Physical Electronics (5400 PHI ESCA) spectrometer with a monochromatic Al Kα X-ray source operated at 300 W and base pressure of 1 × 10−9 Torr. The binding energy of the Au (4f7/2) at 84.0±0.1 eV was used to calibrate the binding energy scale of the spectrometer, and ratios of Ce3+ and Ce4+ in the samples were determined.62, 63 Results were collected from three or more independent experiments and are expressed as mean±S.D. Statistical analysis of two populations was compared using two-tailed non-paired Student’s t-test.APF live-cell imaging
Nanoceria preparation
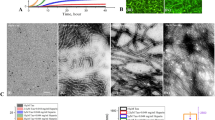
Transmission electron microscopy
X-ray photoelectron spectroscopy
Statistics
Abbreviations
- Aβ:
-
amyloid beta
- AD:
-
Alzheimer’s disease
- APF:
-
3'-(p-aminophenyl) fluorescein
- H2O2:
-
hydrogen peroxide
- CeO2:
-
cerium oxide
- DRP1:
-
dynamin-related protein 1
- DTPA:
-
diethylenetriaminepentaacetic acid
- NAC:
-
N-acetyl-L-cysteine
- NMDA:
-
N-methyl D-aspartate
- NO:
-
nitric oxide
- 3-NP:
-
3-nitropropionic acid
- 3-NT:
-
3-nitrotyrosine
- ROS:
-
reactive oxygen species
- RNS:
-
reactive nitrogen species
- RT:
-
room temperature
- SNOC:
-
S-nitrosocysteine
- SOD:
-
superoxide dismutase
References
Knott AB, Bossy-Wetzel E . Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal 2009; 11: 541–554.
Beal MF . Oxidatively modified proteins in aging and disease. Free Radic Biol Med 2002; 32: 797–803.
Good PF, Werner P, Hsu A, Olanow CW, Perl DP . Evidence of neuronal oxidative damage in Alzheimer's disease. Am J Pathol 1996; 149: 21.
Guix FX, Ill-Raga G, Bravo R, Nakaya T, de Fabritiis G, Coma M et al. Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain 2009; 132 (Pt 5): 1335–1345.
Guix FX, Wahle T, Vennekens K, Snellinx A, Chavez-Gutierrez L, Ill-Raga G et al. Modification of gamma-secretase by nitrosative stress links neuronal ageing to sporadic Alzheimer's disease. EMBO Mol Med 2012; 4: 660–673.
Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron 2011; 71: 833–844.
Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G . Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci 1997; 17: 2653–2657.
Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, **a Z et al. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol 2000; 150: 335–347.
Stamler JS, Lamas S, Fang FC . Nitrosylation. the prototypic redox-based signaling mechanism. Cell 2001; 106: 675–683.
Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ . Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem 2000; 75: 1878–1888.
Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 2004; 41: 351–365.
Cuajungco MP, Lees GJ . Nitric oxide generators produce accumulation of chelatable zinc in hippocampal neuronal perikarya. Brain Res 1998; 799: 118–129.
Yamamoto T, Maruyama W, Kato Y, Yi H, Shamoto-Nagai M, Tanaka M et al. Selective nitration of mitochondrial complex I by peroxynitrite: involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J Neural Transm 2002; 109: 1–13.
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E . Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 2008; 9: 505–518.
Swerdlow RH, Burns JM, Khan SM . The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis 2010; 20 (Suppl 2): S265–S279.
Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 2006; 25: 3900–3911.
Gan X, Huang S, Wu L, Wang Y, Hu G, Li G et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer's disease cybrid cell. Biochim Biophys Acta 2014; 1842: 220–231.
Manczak M, Calkins MJ, Reddy PH . Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet 2011; 20: 2495–2509.
Smirnova E, Griparic L, Shurland DL, van der Bliek AM . Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 2001; 12: 2245–2256.
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 2009; 324: 102–105.
Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB et al. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. J Alzheimers Dis 2010; 20 (Suppl 2): S513–S526.
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 2009; 29: 9090–9103.
Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K . Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 2007; 282: 11521–11529.
Strack S, Wilson TJ, Cribbs JT . Cyclin-dependent kinases regulate splice-specific targeting of dynamin-related protein 1 to microtubules. J Cell Biol 2013; 201: 1037–1051.
Crews L, Masliah E . Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet 2010; 19: R12–R20.
Murakami K, Murata N, Noda Y, Tahara S, Kaneko T, Kinoshita N et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of Alzheimer disease. J Biol Chem 2011; 286: 44557–44568.
Richardson JS . Free radicals in the genesis of Alzheimer's Disease. Ann N Y Acad Sci 1993; 695: 73–76.
Esposito L, Raber J, Kekonius L, Yan F, Yu G-Q, Bien-Ly N et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci 2006; 26: 5167–5179.
Massaad CA, Washington TM, Pautler RG, Klann E . Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 13576–13581.
Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P et al. Electron localization determines defect formation on ceria substrates. Science 2005; 309: 752–755.
Heckert EG, Karakoti AS, Seal S, Self WT . The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008; 29: 2705–2709.
Korsvik C, Patil S, Seal S, Self WT . Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun 2007; 10: 1056–1058.
Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb) 2010; 46: 2736–2738.
Dowding JM, Dosani T, Kumar A, Seal S, Self WT . Cerium oxide nanoparticles scavenge nitric oxide radical (NO). Chem Commun (Camb) 2012; 48: 4896–4898.
Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med 2011; 51: 1155–1163.
Singh S, Kumar A, Karakoti A, Seal S, Self WT . Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst 2010; 6: 1813–1820.
Karakoti AS, Munusamy P, Hostetler K, Kodali V, Kuchibhatla S, Orr G et al. Preparation and characterization challenges to understanding environmental and biological impacts of nanoparticles. Surf Interface Anal 2012; 44: 882–889.
Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E . Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ 2009; 16: 899–909.
Chaturvedi RK, Beal MF . Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci 2008; 1147: 395–412.
Gilgun-Sherki Y, Melamed E, Offen D . Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001; 40: 959–975.
Frei B . Efficacy of dietary antioxidants to prevent oxidative damage and inhibit chronic disease. J Nutr 2004; 134: 3196S–3198S.
Amin KA, Hassan MS, Awad el ST, Hashem KS . The protective effects of cerium oxide nanoparticles against hepatic oxidative damage induced by monocrotaline. Int J Nanomed 2011; 6: 143–149.
Tohgi H, Abe T, Yamazaki K, Murata T, Ishizaki E, Isobe C . Alterations of 3-nitrotyrosine concentration in the cerebrospinal fluid during aging and in patients with Alzheimer's disease. Neurosci Lett 1999; 269: 52–54.
Pacher P, Beckman JS, Liaudet L . Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87: 315–424.
Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G et al. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem 2007; 282: 37006–37015.
Shibuya A, Wada K, Nakajima A, Saeki M, Katayama K, Mayumi T et al. Nitration of PPARgamma inhibits ligand-dependent translocation into the nucleus in a macrophage-like cell line, RAW 264. FEBS Lett 2002; 525: 43–47.
Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007; 28: 1918–1925.
Schubert D, Dargusch R, Raitano J, Chan SW . Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun 2006; 342: 86–91.
Chen J, Patil S, Seal S, McGinnis JF . Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol 2006; 1: 142–150.
Zhou X, Wong LL, Karakoti AS, Seal S, McGinnis JF . Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mouse. PLoS One 2011; 6: e16733.
Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE . Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res 2007; 73: 549–559.
Arnold MC, Badireddy AR, Wiesner MR, Di Giulio RT, Meyer JN . Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Arch Environ Contam Toxicol 2013; 65: 224–233.
Cheng G, Guo W, Han L, Chen E, Kong L, Wang L et al. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways. Toxicol In Vitro 2013; 27: 1082–1088.
Kumari M, Singh SP, Chinde S, Rahman MF, Mahboob M, Grover P . Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int J Toxicol 2014; 33: 86–97.
Ristow M, Schmeisser S . Extending life span by increasing oxidative stress. Free Radic Biol Med 2011; 51: 327–336.
Heckman KL, DeCoteau W, Estevez A, Reed KJ, Costanzo W, Sanford D et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 2013; 7: 10582–10596.
Kim CK, Kim T, Choi IY, Soh M, Kim D, Kim YJ et al. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl 2012; 51: 11039–11043.
Yokel RA, Au TC, MacPhail R, Hardas SS, Butterfield DA, Sultana R et al. Distribution, elimination, and biopersistence to 90 days of a systemically introduced 30 nm ceria-engineered nanomaterial in rats. Toxicol Sci 2012; 127: 256–268.
Karakoti AS, Das S, Thevuthasan S, Seal S . PEGylated inorganic nanoparticles. Angew Chem Int Ed Engl 2011; 50: 1980–1994.
Song W, Bossy B, Martin OJ, Hicks A, Lubitz S, Knott AB et al. Assessing mitochondrial morphology and dynamics using fluorescence wide-field microscopy and 3D image processing. Methods 2008; 46: 295–303.
Patil S, Kuiry SC, Seal S, Vanfleet R . Synthesis of nanocrystalline ceria particles for high temperature oxidation resistant coating. J Nanopart Res 2002; 4: 433–438.
Burroughs P, Hamnett A, Orchard AF, Thornton G . Satellite structure in X-Ray photoelectron-spectra of some binary and mixed oxides of lanthanum and cerium. J Chem Soc Dalton Trans 1976; 17: 1686–1698.
Romeo M, Bak K, Elfallah J, Lenormand F, Hilaire L . Xps study of the reduction of cerium dioxide. Surf Interface Anal 1993; 20: 508–512.
Acknowledgements
We thank ** Chen, Sarah Lubitz, Viviana DeAssis, Cory Eldon and Brad Kincaid for their technical assistance; Mason Mackey, NCMIR, UCSD for assistance with EELS; and Andrew Knott for manuscript editing and development. This work is supported by NIH grants R01NS047456, R01EY016164 and R01NS055193 (to EB-W); NIH grants 5P41RR004050, P41GM103412-24, P42ES010337 and P01DK54441 (to ME); NIH grant R01AG031529-01 (to WTS and SS); and NSF grant CBET 0708172 (to SS and WTS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Deshmukh
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Dowding, J., Song, W., Bossy, K. et al. Cerium oxide nanoparticles protect against Aβ-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ 21, 1622–1632 (2014). https://doi.org/10.1038/cdd.2014.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.72
- Springer Nature Limited
This article is cited by
-
Biosafe cerium oxide nanozymes protect human pluripotent stem cells and cardiomyocytes from oxidative stress
Journal of Nanobiotechnology (2024)
-
Nano biomaterials based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: an up-to-date perspective
Journal of Nanobiotechnology (2023)
-
Synergistic effect of cerium and structure directing agent on drug release behavior and kinetics
Journal of Sol-Gel Science and Technology (2023)
-
Intranasal inorganic cerium oxide nanoparticles ameliorate oxidative stress induced motor manifestations in haloperidol-induced parkinsonism
Inflammopharmacology (2023)
-
Nanomaterials alleviating redox stress in neurological diseases: mechanisms and applications
Journal of Nanobiotechnology (2022)