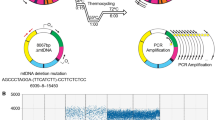
Abstract
Extra-long PCR (XL-PCR) was used to assess the relative concentration of functional full-length mitochondrial DNA (mtDNA) in single type I human vastus lateralis muscle fibres of defined cytochrome c oxidase (COX)activity. Type I muscle fibres rely more on mitochondrial oxidative phosphorylation for their energy demands, compared to the other common fibre types (IIa, IIab and IIb) that principally depend on glycolysis for their energy requirements. A total of 60 single type I fibres were analyzed from 15 individuals (8males and 7 females) of various ages. COX positive muscle fibres were shown to contain amplifiable full-length mtDNA together with a small number of mtDNA rearrangements. By contrast, COX negative fibres did not contain detectable full-length mtDNA, but did contain aheterogeneous mixture of rearranged mtDNA species with the frequency and occurrence of each deletion varying considerably from fibre to fibre. These data lead us to the conclusion that the level of COX activity in type I muscle fibres is reflected by the amount of amplifiable full-length mtDNA. It is proposed that the amount of amplifiable full-length mtDNA constitutes the functional fraction of the total mtDNA. A comprehensive hypothesis that relates the dynamics of mtDNA turnover, mtDNA mutations, mtDNA damage and repair to the ageing process is discussed.
Similar content being viewed by others
References
Aoyagi Y and Shephard RJ (1992) Aging and muscle function. Sports Med 14: 376-396
Birky CW Jr (1983) Relaxed cellular controls and organelle heredity. Science 222: 468-475
Bohr VA and Anson RM (1999) Mitochondrial DNA repair pathways. J Bioenerg Biomembr 31: 391-398
Borges O and Essén-Gustavsson B (1989) Enzyme activities in type I and II muscle fibres of human skeletal muscle in relation to age and torque development. Acta Physiol Scand 136: 29-36
Brooks SV and Faulkner JA (1994) Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc 26: 432-439
de Lacalle S, Iraizoz I and Gonzalo LM (1991) Differential changes in cell size and number in topographic subdivisions of human basal nucleus in normal aging. Neuroscience 433: 445-456
Essén B, Jansson E, Henriksson J, Taylor A and Saltin W (1975) Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand 95: 153-165
Fischer W, Chen KS, Gage FH and Bjorklund A (1991) Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging 13: 9-23
Frischknecht R (1998) Effect of training on muscle strength and motor function in the elderly. Reprod Nutr Dev 38: 167-174
Gadaleta MN, Petruzzella V, Daddabbo L, Olivieri C, Fracasso F, Loguercio Polosa P and Cantatore P (1994) Mitochondrial DNA transcription and translation in aged rat. Effect of acetyl-L-carnitine. Ann N Y Acad Sci USA 717: 150-160
Gross NJ, Getz GS and Rabinowitz M (1969) Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem 244: 1552-1562
Kai Y, Miyako K, Muta T, Umeda S, Irie T, Hamasaki N, Takeshige K and Kang D (1999) Mitochondrial DNA replication in human T lymphocytes is regulated primarily at the H-strand termination site. Biochim Biophys Acta 1446: 126-134
Kopsidas G, Kovalenko SA, Kelso JM and Linnane AW (1998) An age-associated correlation between cellular bioenergy decline and mtDNA rearrangements in human skeletal muscle. Mutat Res 421: 27-36
Kopsidas G, Kovalenko SA, Islam MM, Gingold EB and Linnane AW (2000a) Preferential amplification is minimised in long-PCR systems. Mutat Res 456: 83-88
Kopsidas G, Kovalenko SA, Heffernan DR, Yarovaya N, Kramarova L, Stojanovski D, Borg J, Islam MM, Caragounis A and Linnane AW (2000b) Tissue mitochondrial DNA changes. A stochastic system. Ann NY Acad Sci 908: 226-243
Kovalenko SA, Harms PJ, Tanaka M, Baumer A, Kelso J, Ozawa T and Linnane AW (1997a) Method for in situ investigation of mitochondrial DNA deletions. Hum Mutat 10: 489-495
Kovalenko SA, Kopsidas G, Kelso JM and Linnane AW (1997b) Deltoid human muscle mtDNA is extensively rearranged in old age subjects. Biochem Biophys Res Commun 232: 147-152
Kovalenko SA, Kopsidas G, Kelso JM, Rosenfeldt FL and Linnane AW (1998a) Tissue-specific distribution of multiple mitochondrial DNA rearrangements during human aging. Annal NY Acad Sci 854: 171-182
Kovalenko SA, Kopsidas G, Islam M, Heffernan DR, Fitzpatric JA, Caragounis A, Gingold, EB and Linnane AW (1998b) The age-associated decrease in the amount of amplifiable full-length mitochondrial DNA in human skeletal muscle. Biochem Mol Biol Internat 46: 1233-1241
Linnane AW, Zhang C, Baumer A and Nagley P (1992) Mitochondrial DNA mutation and the ageing process: bioenergy and pharmacological intervention. Mutat Res 275: 195-208
Linnane AW, Kopsidas G, Kelso JM and Kovalenko SA (1997) Occurence of age-associated multiple mtDNA rearrangments in human skeletal muscle. Fed American Soc Exp Biol J 11: A1450
Olivetti G, Melissari M, Capasso JM and Anversa P (1991) Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 68: 1560-1568
Ozawa T (1994) Mitochondrial cardiomyopathy. Herz 19: 105-118
Roberti M, Musicco C, Polosa PL, Milella F, Gadaleta MN and Cantatore P (1998) Multiple protein-binding sites in the TASregion of human and rat mitochondrial DNA. Biochem Biophys Res Commun 243: 36-40
Shadel GS and Clayton DA (1997) Mitochondrial DNA maintenance in vertebrates. Annu Rev Biochem 66: 409-435
Stroessner-Johnson HM, Rapp PR and Amaral DG (1992) Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged rhesus monkey. J Neurosci 12: 1936-1944
Wallace DC (1994) Mitochondrial DNA mutations in diseases of energy metabolism. J Bioenerg Biomembr 26: 241-250
Wei YH, Lu CY, Lee HC, Pang CY and Ma YS (1998) Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann NY Acad Sci 854: 155-170
Yakes FM and Van Houten B (1997) Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94: 514-519
Yarovaya NO, Kramarova L, Borg J, Kovalenko SA, Caragounis A and Linnane A (2002) Age-related atrophy of rat soleus muscle is accompanied by changes in fibre type composition, bioenergy decline and mtDNA rearrangements. Biogerontology 3: 25-27 (this issue)
Zhang C, Linnane AW and Nagley P (1993) Occurrence of a particular base substitution (3243 A to G) in mitochondrial DNA of tissues of ageing humans. Biochem Biophys Res Commun 195: 1104-1110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kopsidas, G., Zhang, C., Yarovaya, N. et al. Stochastic mitochondrial DNA changes: bioenergy decline in type I skeletal muscle fibres correlates with a decline in the amount of amplifiable full-length mtDNA. Biogerontology 3, 29–36 (2002). https://doi.org/10.1023/A:1015290810222
Issue Date:
DOI: https://doi.org/10.1023/A:1015290810222