Abstract
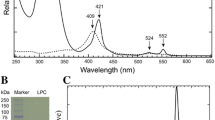
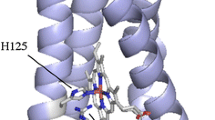
To understand the roles of negatively surface charged residues, the cytochrome b5 (Cyt b5) E48A/D60A mutant was constructed. UV-visible and CD spectra confirmed that the mutation did not cause overall structural changes of the protein. The mutant presents an unexpected high stability toward the thermal and denaturant compared with the wild type Cyt b5, which shows that these surface charged residues can influence the interactions between the heme b group and the polypeptide chain. Functional properties were clarified through the electron transfer reactions between Cyt b5 and Cyt c. The driving force of the electron transfer reactions is conservative. Although the association constant of Cyt b5 E48A/D60A with Cyt c is much lower than that of the wild type Cyt b5, their electron transfer rate constants do not differ significantly. The results show that these surface charged residues play important roles in regulating both the stability and functional properties of Cyt b5.
Similar content being viewed by others
REFERENCES
Baymann, F., Moss, D. A., and Mantele, W. (1991). Anal.Biochem. 199, 269–274.
Bernardi, P. and Azzone, G. F. (1981). J.Biol.Chem. 256, 7187–7192.
Caffrey, M. S. (1994). Biochimie 76, 622–630.
Caffrey, M. S. and Cusanovich, M. A. (1991). Arch.Biochem.Biophys. 258, p227–230.
Caffrey, M. S. and Cusanovich, M. A. (1994). Biochim.Biophys.Acta 1187, 277–288.
Davidson, V. L. (2000). Acc.Chem.Res. 33, 87–93.
Estabrook, R. W., Hildebrandt, A. G., Baron, J., Nettar, K. J., and Leibman, K. (1971). Biochem.Biophys.Res.Commun. 42, 132–139.
Funk, W. D., Lo, T. P., Mauk, M. R., Braver, G. D., MacGillivray, A. M., and Mauk, A. G. (1990). Biochemistry 29, 5500–5508.
Hewson, R., Newbold, R. J., and Whitford, D. (1993). Protein Eng. 6, 953–964.
Kessler, D. L., Johnson, J. L., Cohen, H. J., and Rajagopalan, K. U. (1974). Biochem.Biophys.Acta 334, 86–96.
Loladze, V. V., Ibarra-Molero, B., Sanchez-Ruiz, J. M., and Makhatadze, G. I., (1999). Biochemistry 38, 16419–16423.
Manyusa, S. and Whitford, D. (1999). Biochemistry 38, 9533–9540.
Mathews, B. (1993). Annu.Rev.Biochem. 63, 139–160.
Mauk, A. G., Mauk, M. G., Moore, G. R., and Northrup, S. H., (1995). J.Bioenerg.Biomembr. 27, 311–330.
Mauk, M. R., Reid, L. S., and Mauk, A. G. (1982). Biochemistry 24, 1843–1846.
Newbold, R. J., Hewson, R., and Whitford, D. (1992). FEBS Lett. 314, 419–424.
Northrup, S. H., Thomassm, K. A., Miller, C. M., Barker, P. D., Geltis, L. D., Guillemette, J. G., Inglis, S. C., and Mauk, A. G. (1993). Biochemistry 32, 6613–6623.
Pfeil, W. (1993). Protein Sci. 2, 1497–1501.
Poulos, T. L. (1988). Adv.Inorg.Biochem. 7, 1–36.
Qian, W., Sun, Y. L., Wang, Y. H., Zhuang, J. H., **e, Y., and Huang, Z. X. (1998). Biochemistry 37, 14137–14150.
Saburo, N., Hiroshi, H., et al. (1997). J.Biochem. 121, 654–660.
Salemme, F. R. (1976). J.Mol.Biol. 102, 563–568.
Sambrook, J., Fristch, E. F., and Maniatis, T. (1989). In Molecular Cloning: A Labrotory Manual (2nd ed.). Cold Spring Harbor Laboratory Press: Plainview, New York, pp. 51–80.
Sannes, L. J. and Hultquist, D. E. (1978). Biochem.Biophys.Acta 544, 547–554.
Strittmatter, P., Spatz, L., Crcoran, D., Rogers, M. J., Setlow, B., and Redline, R. (1974). Proc.Nat.Acad.Sci.USA 71, 4565–4569.
Sun, Y. L., Wang, Y. H., Yan, M. M., Sun, B. Y., **e, Y., Huang, Z. X., Jiang, S. K., and Wu, H. M. (1999). J.Mol.Biol. 285, 347–359.
Wang, Y. H., Cui, J., Sun, Y. L., Yao, P., Zhuang, J. H., and **e, Y. (1997). J.Electroanal.Chem. 428, 39–45.
Wang, Z. Q., Wang, Y. H., Wang, W. H., Xue, L. L., Wu, X. Z., **e, Y., and Huang, Z. X. (2000). Biophys.Chem. 83, 3–17
Xue, L. L., Wang, Y. H., **e, Y., Yao, P., Wang, W. H., Qian, W., and Huang, Z. X. (1999). Biochemistry 38, 11961–11972.
Yao, P., **e, Y., Wang, Y. H., Sun, Y. L., Huang, Z. X., **ao, G. T., and Wang, S. D. (1997). Protein Eng. 10, 575–581.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, YH., Ren, Y., Wang, WH. et al. The Regulation of Surface Charged Residues on the Properties of Cytochrome b5. J Protein Chem 20, 487–493 (2001). https://doi.org/10.1023/A:1012506513521
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1012506513521