Abstract
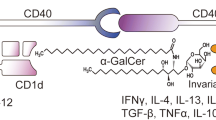
The combined therapeutic effect of natural killer T (NKT) cell ligand α-galactosylceramide (α-GalCer) and IL-12 against highly metastatic B16-BL6-HM melanoma cells was investigated. In comparison with a single administration of α-GalCer or IL-12, the combined treatment of tumor-bearing mice with α-GalCer plus IL-12 caused a super-induction of serum IFN-γ levels, though α-GalCer-induced IL-4 production was rather inhibited. In parallel with the augmented IFN-γ production, the natural killing activity against YAC-1 cells and syngeneic B16- BL6-HM melanoma was greatly augmented by the combined therapy. The major effector cells responsible for natural killing activity induced by α-GalCer plus IL- 12 were enriched in both NK1.1+TCRαβ+ NKT cells and NK1.1+TCRαβ− NK cells. The preventing effect of α-GalCer or IL-12 alone against lung metastasis of B16-BL6-HM was also enhanced by the combination therapy. The antitumor activity of α-GalCer was totally abolished in NKT-deficient mice. However, IL- 12-induced antitumor activity was not eliminated in NKT-deficient mice though it was inhibited by anti-asialo GM1 Ab treatment. These findings suggested that α-GalCer synergistically act with IL-12 to activate both NKT cells and NK cells, which may play a critical role in the strong prevention of distant tumor metastasis at early stages of tumor-bearing. These data will provide a novel tool for the prevention of tumor metastasis using NKT-specific ligands α-GalCer and IL-12.
Similar content being viewed by others
References
Koseki H, Imai K, Nakayama F et al. Homogenous junctional sequence of the V+14C T-cell antigen receptor α chain expanded in unprimed mice. Proc Natl Acad Sci USA 1990; 87(14): 5248–52.
Fowlkes BJ, Kruisbeek AM, Ton-That H et al. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly express a single Vβ gene family. Nature 1987; 329(6136): 251–4.
Ohteki T, MacDonald HR. Stringent Vβ requirement for the development of NK1.1+ T cell receptor-α/β + cells in mouse liver. J Exp Med 1996; 183(3): 1277–82.
Arase H, Arase N, Ogasawara K et al. An NK1.1+ CD4+8- singlepositive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor Vβ family. Proc Natl Acad Sci USA 1992; 89(14): 6506–10.
Bendelac A, Rivera MN, Park SH et al. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol 1997; 15: 535–62.
Yankelevich B, Knobloch C, Nowicki M et al. A novel cell type responsible for bone marrow graft regection in mice. T cell with NK phenotype cause acute rejection of bone marrow grafts. J Immunol 1989; 142: 3423–30.
Ballas ZK, Rasmussen K. NK1.1C thymocyte. Adult murine CD4- CD4-, CD8- thymocytes contain an NK1.1+, CD3C, CD5hi, CD44hi, TCRVβ8+ subset. J Immunol 1990; 145(4): 1039–45.
Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8- and CD4-8- subset of natural killer 1.1+ T cell receptor-α/β + cells in the liver of mice. J Exp Med 1994; 180(2): 699–704.
Sykes M. Unusual T cell population in adult murine bone marrow. Prevalence of CD3+CD4-CD8- and α/β TCR+NK1.1+ cells. J Immunol 1990; 145(10): 3209–15.
Levisky H, Golumbek P, Pardoll D. The fate of CD4-CD8- T cell receptor α/β + thymocytes. J Immunol 1991; 146(4): 1113–7.
Bendelac A, Lantz O, Quimby ME et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995; 268(5212): 863–5.
Porcelli S, Yockey CE, Brenner MB et al. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- α/β T cells demonstrales preferential use of several Vβ genes and an invariant TCR α chain. J Exp Med 1993, 178(1): 1–16.
Kawano T, Cui J, Koezuka Y et al. CD1d-restricted and TCRmediated activation of Vα 14 NKT cells by glycosylceramides. Science (Washington DC) 1997; 278(5343): 1626–9.
Kawano T, Tanaka Y, Shimizu E et al. A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int Immunol 1999, 11(6): 881–7.
Kitamura H, Iwakabe K, Yahata T et al. The natural killer T (NKT) cell ligand a-Galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med 1999; 189(7): 1121–8.
Singh N, Hong S, Scerer DC et al. Activation of NKT cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol 1999; 163(5): 2373–7.
Burdin N, Brossay L, Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NKT cells towards Th2 cytokine synthesis. Eur J Immunol 1999, 29(6): 2014–25.
Nakui M, Iwakabe K, Ohta A et al. Natural killer T cell ligand α-galactosylceramide inhibited lymph node metastasis of highly metastatic melamoma cells. Jpn J Cancer Res 1999; 90(8): 801–4.
Kobayashi E, Motoki K, Uchida T et al. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 1995; 7(10-11): 529–34.
Motoki K, Maeda K., Ueno H et al. Antitumor activities of combined treatment with a novel immunomodulator, (2S,3S,4R)-1-O-(α-D-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol (KRN7000), and radiotherapy in tumor-bearing mice. Oncol Res 1996; 8(4): 155–62.
Nishimura T., Santa K, Yahata T et al. Involvement of IL-4-producing Vβ8.2+ CD4+ CD62L- CD45RB- T cells in non-MHC genecontrolled predisposition toward skewing into T helper type-2 immunity in BALB/c mice. J Immunol 1997; 158(12): 5698–706.
Sato N, Yahata T, Santa K et al. Functional characterization of NK1.1+ Ly-6C+ cells. Immunol Lett 1996; 54(1): 5–9.
Nishimura T, Burakoff SJ, Herrmann SH. Protein kinase C required for cytotoxic T lymphocyte triggering. J Immunol 1987; 139(9): 2888–91.
Carnaud C, Lee D, Donnars O et al. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol 1999; 163(9): 4647–50.
Kawano T, Cui J, Koezuka Y et al. Natural killer like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acd Sci USA 1998; 95(10): 5690–3.
Hashimoto W, Takeda K, Anzai R et al. Cytotoxic NK1.1 Ag+ α/β T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol 1995; 154(9): 4333–40.
Rorsenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am 2000; Suppl 1, S2–7.
Vetto JT, Papa MZ, Lotze MT et al. Reduction of toxicity of interleukin-2 and lymphokine-activated killer cells in humans by the administration of corticosteroids. J Clin Oncol 1987; 5(3): 496–503.
Nishimura T, Togashi Y, Goto M et al. Augmentation of the therapeutic efficacy of adoptive tumor immunotherapy by in vivo administration of slowly released recombinant interleukin 2. Cancer Immunol Immunother 1986; 21(1): 12–8.
Takeda K, Hayakawa Y, Atsuta M et al. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol 2000; 12(6): 909–14.
Nakagawa R, Motoki K, Ueno H et al. Treatment of Hepatic metastasis of Colon26 adenocarcinoma with an α-galactosylceramide, KRN7000. Cancer Res 1998; 58(6): 1202–7.
Kawamura T, Takeda K, Mendiratta SK et al. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J Immunol 1998; 160(1): 16–9.
Cui, J., Shin T, Kawano T et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 1997; 278(5343): 1623–6.
Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996; 17(3): 138–146.
Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 and Th2 cytokines. Immunity 1995; 2(3): 271–9.
Sato M, Iwakabe K, Kimura S et al. Functional skewing of bone marrow-derived dendritic cells by Th1-or Th2-inducing cytokines. Immunol Lett 1999, 67(1): 63–8.
Nishimura T, Kitamura H, Iwakabe K et al. The interface between innate and aquired immunity. Glycolipid antigen presentation by CD1d-expressing dendritic cells to natural killer T cells induces the differenciation of antigen-specific cytotoxic T lymphocytes. Int Immunol 2000; 12(7): 987–94.
Nishimura T, Iwakabe K, Sekimoto M et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med 1999; 190(5): 617–27.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakui, M., Ohta, A., Sekimoto, M. et al. Potentiation of antitumor effect of NKT cell ligand, α-galactosylceramide by combination with IL-12 on lung metastasis of malignant melanoma cells1. Clin Exp Metastasis 18, 147–153 (2000). https://doi.org/10.1023/A:1006715221088
Issue Date:
DOI: https://doi.org/10.1023/A:1006715221088