Abstract
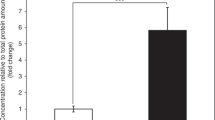
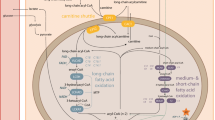
Mitochondrial fatty acid oxidation disorders cause hypoglycaemia, hepatic dysfunction, myopathy, cardiomyopathy and encephalopathy. Despite their recognition for more than 15 years, diagnosis and treatment remain difficult. To help design rational diagnostic and therapeutic strategies, we studied the pathophysiology of accumulating metabolites in a whole-cell system. Acylcarnitines were quantified in cells and media of cultured fibroblasts after incubation with L-carnitine and fatty acids. Following incubation with palmitate, long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD)-deficient fibroblasts compared with controls showed elevation of hydroxypalmitoyl- and palmitoylcarnitine and reduction of C10- and shorter acylcarnitines, and following incubation with linoleate an increase in C14:2-, C18:2- and hydroxy-C18:2-acylcarnitines and reduction in C10:1-acylcarnitines. Hydroxyacylcarnitines remained more intracellular compared to corresponding saturated acylcarnitines. Incubation with decanoate and octanoate showed absence of hydroxylated acylcarnitines and correction of secondary metabolic disturbances, suggesting that optimal treatment should include medium-chain triglycerides of these chain lengths. Fibroblasts of patients with other fatty acid oxidation disorders showed distinct elevations of disease-specific acylcarnitines. This acylcarnitine analysis allows the diagnosis of LCHAD deficiency and its differentiation from other fatty acid oxidation disorders, which can pose difficulties in vivo. The strategy has allowed in-depth analysis with different substrates, providing suggestions for the rational design of treatment trials.
Similar content being viewed by others
REFERENCES
Bohmer T, Behmer J (1958) Propionylcarnitine, physiological variations in vivo. Biochim Biophys Acta 152: 559-567.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72: 248-254.
Carpenter K, Middleton B, Pollitt R (1992a) Purification of long-chain 3-hydroxyacyl-CoA dehydrogenase from human infant liver. In Coates PM, Tanaka K, eds. Progress in Clinical and Biological Research, 375: New Developments in Fatty Acid Oxidation. New York: Wiley-Liss, 75-84.
Carpenter K, Pollitt RJ, Middleton B (1992b) Human liver long-chain 3-hydroxyacylcoenzyme A dehydrogenase is a multifunctional membrane-bound beta-oxidation enzyme of mitochondria. Biochem Biophys Res Commun 183: 443-448.
Costa CG, Dorland L, Holwerda U, et al (1998) Simultaneous analysis of plasma free fatty acids and their 3-hydroxy analogs in fatty acid β-oxidation disorders. Clin Chem 44: 463-471.
Duran M, Wanders RJA, deJager JP, et al (1991) 3-Hydroxydicarboxylic aciduria due to long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deffciency associated with sudden neonatal death: protective effect of medium-chain triglyceride treatment. Eur J Pediatr 150: 190-195.
Eaton S, Bartlett K, Pourfarzam M (1996) Mammalian mitochondrial β-oxidation. Biochem J 320: 345-357.
El-Fakhri M, Middleton B (1982) The existence of an inner-membrane-bound, long acylchain-specific 3-hydroxyacyl-CoA dehydrogenase in mammalian mitochondria. Biochim Biophys Acta 713: 270-279.
Gillingham M, Van Calcar S, Ney D, Wol. J, Harding C (1999) Dietary management of long-chain 3-hydroxyacyl-CoA dehydrogenase deffciency (LCHADD). A case report and survey. J Inher Metab Dis 22: 123-131.
Hagenfeldt L, von Dobeln U, Holme E, et al (1990) 3-Hydroxydicarboxylic aciduria-a fatty acid oxidation defect with severe prognosis. J Pediatr 116: 387-392.
He X-Y, Yang S-Y, Schulz H (1992) Inhibition of enoyl-CoA hydratase by long-chain L-3-hydroxyacyl-CoA and its possible effect on fatty acid oxidation. Arch Biochem Biophys 298: 527-531.
Ijlst L, Ruiter JPN, Hoovers JMN, Jakobs ME, Wanders RJA (1996) Common missense mutation G1528C in long-chain 3-hydroxyacyl-CoA dehydrogenase deffciency. J Clin Invest 98: 1028-1033.
Kler RS, Jackson S, Bartlett K, et al (1991) Quantitation of acyl-coA and acylcarnitine esters accumulated during abnormal mitochondrial fatty acid oxidation. J Biol Chem 266: 22932-22938.
Kobayashi A, Jiang LL, Hashimoto T (1996) Two mitochondrial 3-hydroxyacyl-CoA dehydrogenase in bovine liver. J Biochem 119: 775-782.
Libert R, Van Hoof F, Thillaye M, et al (1997) Identification of new medium-chain acylcarnitines present in normal human urine. Anal Biochem 251: 196-205.
Luo MJ, He X-Y, Sprecher H, Schulz H (1993) Purification and characterization of the trifunctional β-oxidation complex from pig heart mitochondria. Arch Biochem Biophys 304: 266-271.
Millington DS, Chace DH (1992) Carnitine and acylcarnitines in metabolic disease diagnosis and management. In Desiderio DM, ed. Mass Spectrometry: Clinical and Biomedical Applications, Vol. I. New York: Plenum Press, 299-318.
Millington DS, Norwood DL, Kodo N, Roe CR, Inuoe F (1989) Application of fast atom bombardment with tandem mass spectrometry and liquid chromatography/mass spectrometry to the analysis of acylcarnitines in human urine, blood, and tissue. Anal Biochem 180: 331-339.
Morris AAM, Shortland GJ, van Wijk K, et al (1998) Use of medium-chain triglyceride in long-chain 3-hydroxyacyl-CoA dehydrogenase deffciency. J Inher Metab Dis 21(supplement 3): 3 (Abstract).
Nada MA, Chace DH, Sprecher H, Roe CR (1995a) Investigation of β-oxidation in normal and MCAD-deficient human fibroblasts using tandem mass spectrometry. Biochem Mol Med 54: 59-66.
Nada MA, Rhead WJ, Sprecher H, Schulz H, Roe CR (1995b) Evidence for intermediate channeling in mitochondrial β-oxidation. J Biol Chem 270: 530-535.
Pons R, Roig M, Riudor E, et al (1996) The clinical spectrum of long-chain 3-hydroxyacyl-CoA dehydrogenase deffciency. Pediatr Neurol 14: 236-243.
Pourfarzam M, Schaefer J, Turnbull DM, Bartlett K (1994) Analysis of fatty acid oxidation intermediates in cultured fibroblasts to detect mitochondrial oxidation disorders. Clin Chem 40: 2267-2275.
Roe CR, Coates PM (1995) Mitochondrial fatty acid oxidation disorders. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease, 7th edn. New York: McGraw-Hill, 1501-1533.
Schaefer J, Pourfarzam M, Bartlett K, Jackson S, Turnbull DM (1995) Fatty acid oxidation in peripheral blood cells: characterization and use for the diagnosis of defects of fatty acid oxidation. Pediatr Res 37: 354-360.
Thorpe C (1990) The reductive half reaction in the acyl-CoA dehydrogenases. In Tanaka K, Coates P, eds. Progress in Clinical and Biological Research, 321 Fatty Acid Oxidation: Clinical, Biochemical, and Molecular Aspects. New York: Alan R. Liss, 91-105.
Treem WR, Shoup ME, Hale DE, et al (1996) Acute fatty liver of pregnancy, hemolysis, elevated liver enzymes, and low platelets syndrome, and long chain 3-hydroxyacylcoenzyme A dehydrogenase deffciency. Am J Gastroenterol 91: 2293-2300.
Tyni T, Paloti A, Vlinikka L, et al (1997) Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deffciency with the G1528C mutation: clinical presentation of thirteen patients. J Pediatr 130: 67-76.
Uchida Y, Izai K, Orii T, Hashimoto T (1992) Novel fatty acid b-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J Biol Chem 267: 1034-1041.
Van Hove JLK, Kahler SG, Ramakrishna JP, et al (1997) Acylcarnitines in 3-hydroxy-longchain-acyl-CoA dehydrogenase deficiency. Paper presented at the 7th International Congress of Inborn Errors of Metabolism, Vienna, Austria.
Ventura FV, Ijlst L, Ruiter J, et al (1998) Carnitine palmitoyltransferase II specificity towards β-oxidation intermediates. Evidence for a reverse carnitine cycle in mitochondria. Eur J Biochem 253: 614-618.
Verhoeven NM, Roe DS, Kok RM, Wanders RJA, Jakobs C, Roe CR (1998) Phytanic and pristanic acid are oxidized by sequential peroxisomal and mitochondrial reactions in cultured fibroblasts. J L ipid Res 39: 66-74.
Vianey-Saban C, Divry P, Brivet M, et al (1998) Mitochondrial very-long chain acylcoenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin Chim Acta 269: 43-62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shen, J.J., Matern, D., Millington, D.S. et al. Acylcarnitines in fibroblasts of patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and other fatty acid oxidation disorders. J Inherit Metab Dis 23, 27–44 (2000). https://doi.org/10.1023/A:1005694712583
Issue Date:
DOI: https://doi.org/10.1023/A:1005694712583