Abstract
Purpose
To construct an effective prognostic nomogram for patients with clinically localized Acral lentiginous melanoma (ALM).
Methods
This retrospective study included ALM patients from the Surveillance, Epidemiology, and End Results (SEER) database from 2004–2015. The 1686 cases were divided into the training and internal validation cohorts at a ratio of 7:3. The Cox regression analyses were performed to select risk factors for the nomogram. The performances of the nomogram were evaluated using the concordance index (C-index), area under receiver operating characteristic curve (AUC), and calibration plots, and validated in an external multicenter cohort.
Results
Age, gender, race, and stage were significantly associated with overall survival (OS), with a C-index of 0.727 and 0.722 in the training and internal validation groups, respectively. The AUC of 2-,4-, and 6 years were higher than or equal to 0.75. Age, gender, race, site, and stage were related to ALM patients' CSS which have better C-index of 0.791 and 0.774 in the training and internal validation set. The AUC of 2-,4-, and 6- were higher than or equal to 0.80. Both survival calibration curves of 2-,4, and 6-year OS and CSS brought out a good consistency. The external multicenter cohort authenticated the value of the nomogram of OS, with a C-index of 0.657 and an AUC of 0.67, 0.72, and 0.65 at 2, 4, and 6 years. Good consistency was also observed.
Conclusion
A prognostic nomogram was established and validated to help predict the survival of ALM patients and guide individualized treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Melanoma is the leading cause of skin cancer-related deaths [1]. Timely and effective treatment is crucial for improving patients’ prognosis. Adjuvant therapy has been demonstrated to be an effective survival enhancer and is now an essential part of the treatment plan for stage III and stage IV patients after complete resection [2]. For stages I and II, even though the a relatively low risk of metastasis, there are still several patients who later suffer metastasis, which often leads to death by melanoma [3]. Currently, clinical trials have now been conducted on stage II patients and have shown effectiveness in high-risk groups [4, 5]. So, a prognostic assay would be highly beneficial in this scenario as it would enable early-stage, high-risk patients to receive treatment without subjecting all patients to the potential side effects of adjuvant treatments. Studies showed that the lesions from certain anatomical sites (cutaneous melanoma of the back) are associated with a lower 5-year overall survival (OS) rate and higher hazard ratio (HR) compared with cutaneous melanoma of other sites [6,7,8]. As the most common subtype of malignant melanoma in Asians and other non-Caucasian populations [9], acral lentiginous melanoma (ALM) originates from melanocytes on the non-hair-bearing skin and the nail unit. ALM is histologically defined since its growth pattern is characterized by a predominantly confluent single-cell (lentiginous) array of atypical melanocytes along the dermo-epidermal junction. ALM is correlated with greater Breslow depth, more advanced clinical stage at presentation, higher rates of sentinel lymph node metastasis and ulceration as well as worse prognosis [10]. Nevertheless, the prognostic significance of ALM in stage I-III patients is not well explored.
At present, most of the study results can not comprehensively evaluate the prognosis of postoperative ALM patients. In addition, owing to the distinct proportion of melanoma subtypes in Asians and Westerners, conclusions that were drawn from the Western populations still warrant further investigation and validation in a wider range of the population. Therefore, the closest approach may be reached by analyzing the data from the Surveillance, Epidemiology, and End Results (SEER) database to help provide ALM's individual risk stratification information. Among the existing assessment tools, the nomogram is a reliable and widely adopted tool to evaluate risk by integrating clinicopathologic characteristics for oncologic outcomes [11,12,13,14,15] and assist patients along with physicians in making optimal clinical decisions. Based on clinicopathologic characteristics of both patients and tumors, nomograms enable personalized computation of independent risk factors simultaneously and draw the most accurate conclusion to predict the prognosis of a particular patient. Thus, nomograms are convenient, and economical and have been proven to show better predictive ability than the traditional Tumor, Node, Metastasis (TNM) staging system in various types of tumors [16]. Nevertheless, the application of nomograms in ALM is rare.
Herein, we collected the baseline information, clinicopathologic characteristics, and follow-up data of stage I-III ALM patients from the SEER database and performed a comprehensive analysis. This study randomized 1686 patients in a 7:3 ratio to the training cohort and validation cohort. In addition, this prognostic nomogram was also validated by a multicenter external cohort from China.
2 Methods
2.1 Patient selection criteria and follow-up
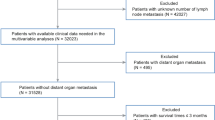
The SEER database (https://seer.cancer.gov/) includes 18 population-based cancers. In this study, all the information for 2160 patients with ALM (#8744) was extracted by the SEER*Stat program (v 8.3.9.2) with diagnosis years from 2004 to 2015 according to the International Classification of Disease for Oncology (ICD‐O) version 3. Patients with the following information were included: Age recodes with < 1-year-olds, Year of diagnosis, Sex, Derived AJCC (American Joint Committee on Cancer) Stage Group, 6th ed (2004–2015), Survival months, Vital status recode, SEER cause-specific death classification. Patients were excluded if any of the data was missing or incomplete or were in stage IV (since all the patients in our cohort are within stage III). According to the standard of the World Health Organization (WHO), using the age of 60 as the, the age was divided into elderly and young groups. Finally, 1686 cases were eligible and randomly allocated in a 7:3 ratio to the training and internal validation cohorts. The training group contained 1181 patients, while 505 patients were in the internal validation group.
In the external validation cohort, patients diagnosed with acral lentiginous melanoma at Fudan University Shanghai Cancer Center (FUSCC, Shanghai, China), Tian** Medical University Cancer Institute and Hospital (TMUCIH, Tian**, China), and Zhejiang Cancer Hospital (ZJCH, Zhejiang, China) between September 1, 2009, and August 31, 2017, were identified. At least two experienced pathologists from each center evaluated all histological specimens. The following variables were also collected: age, gender, initial disease stage and primary anatomical sites, survival months, and vital status. Patient follow-up was performed using re-examination or telephone calls until death or September 31, 2019. Survival was censored at the date of the last follow-up. OS was defined as the time interval from curative resection to death/the last follow-up. Median OS was defined as the time 1/2 the patients are expected to live after curative resection to death/the last follow-up. Cancer-specific survival (CSS) was defined as the duration from the date of diagnosis until death due to acral lentiginous melanoma other than other causes/the last follow-up; Median CSS as the time 1/2 the patients are expected to live after curative resection to death due to acral lentiginous melanoma other than other causes /the last follow-up. Patients with incomplete clinicopathological characteristic data, less than 18 years old, or with a follow-up of less than 1 month after curative resection were excluded. After applying these criteria, 381 patients histologically classified as ALM were identified. This study was approved by the Institutional Review Board and Ethics Committee of the FUSCC, TMUCIH, and ZJCH. Because unidentified health data of patients were used, informed consent was waived by all the institutes mentioned above. All procedures in our study were to the corresponding guidelines and regulations.
2.2 Nomogram construction and validation
Eligible cases from the SEER database were randomly allocated in a 7:3 ratio to the training cohort (n = 1181) or internal validation cohort (n = 505) by using the createDataPartition function in the caret package. In the training group, univariate and multivariate Cox proportional hazards regression models were performed on each variable to investigate the correlation between clinicopathologic features and OS/CSS. The independent prognostic factors were assessed in a multivariate Cox proportional hazards regression with the stepwise selection of variables based on the Akaike information criterion (AIC). Then nomograms (stepwise models) were constructed to predict the probability of 2-, 4- and 6-year OS and CSS probabilities after curative resection in stage I-III ALM patients by utilizing the RMS package in R version 4.1.1 (http://www.r-project.org).
The performance of the constructed nomogram was evaluated by discrimination, calibration, and uniformity. The concordance index (C-index) was used to assess the discrimination ability. The C-index value ranges from 0.5 to 1 and is approximately equivalent to the area under the curve (AUC). C-index and AUC over 0.7 indicate that the nomogram is considered to have a moderate ability to stratify patients into different prognosis groups. Furthermore, receiver operating characteristic (ROC) curves and the corresponding AUCs at 2, 4, and 6 years were also generated to estimate the predictive accuracy. Calibration curves were used to graphically present the uniformity between the predicted probabilities of the nomogram and the observed outcome frequencies. The closer the predicted calibration curve is to the standard curve, the better the predictive ability of the nomogram.
2.3 Statistical analyses
While qualitative variables were described as frequencies and percentages, quantitative variables were described in terms of means and standard deviations (SD). Pearson’s chi-squared test and Student’s t-test were utilized to identify corresponding independent prognostic factors and calculate HRs and 95% CIs from categorical variables and continuous variables. The correlation between clinicopathologic features and prognosis index (OS and CSS) was evaluated by Cox proportional hazards regression models. Derived from the stepwise multivariate analysis, selected risk factors were integrated into the nomogram to predict the probability of 2-, 4- and 6-year OS and CSS probabilities after curative resection in stage I-III ALM patients. All statistical analyses were performed using R software (version 4.1.1, https://www.r-project.org/). The R packages used in this study include caret, survival, survival ROC rms, foreign, survival, nomogramEX, dplyr, and pec. A two-tailed P-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
A total of 1686 ALM patients were extracted from the SEER database from 2004 to 2015 and were randomly allocated in a 7:3 ratio to the training cohort (n = 1181) or internal validation cohort (n = 505). In addition, 381 ALM patients were enrolled as the external validation cohort from the multicenter data (including FUSCC, TMUCIH, and ZJCH) from 2009 to 2017. The flow chart is shown in Fig. 1. In the training cohort, 608 (51.48%) patients were female, and 767 (64.94%) patients were aged ≥ 60 years. In the internal validation cohort, 261 (51.68%) patients were female, and 324 (64.16%) patients were aged ≥ 60 years. In the external validation cohort, 196 (51.44%) patients were female, and 237 (62.20%) patients were aged ≥ 60 years. The demographics and clinicopathologic features of the training, internal, and external validation cohort are presented in Table 1.
3.2 Prognostic risk factors in the training cohort
The univariate analysis indicated that age, gender, and stage were significantly associated with OS (p-value < 0.05), while age, gender, and stage were statistically associated with CSS. Based on the stepwise multivariate COX regression analysis, the following four independent risk factors were selected for the multivariate analysis by using a Cox proportional hazards regression model: age (≥ 60: HR 2.92, 95% CI 2.33–3.67 P < 0.0001), gender (male: HR 1.61, 95% CI 1.35–1.92, P < 0.0001), race (black: HR 1.37, 95% CI 1.02–1.84, P = 0.0388), and stage (IB: HR 1.99, 95% CI 1.46- 2.73, P < 0.0001; IIA: HR 2.03, 95% CI 1.42–2.89, P = 0.0001; IIB: HR 3.21, 95% CI 2.28–4.53, P < 0.0001; IIC: HR 3.46, 95% CI 2.39–5.03, P < 0.0001; IIIA: HR 3.54, 95% CI 2.29–5.45, P < 0.0001; IIIB: HR 4.49, 95% CI 3.19–6.31, P < 0.0001 and IIIC: HR 8.29, 95% CI 5.73–11.98, P < 0.0001). and age (≥ 60: HR 1.93, 95% CI 1.46–2.56, P < 0.0001), gender (male: HR 1.70, 95% CI 1.33–2.18, P < 0.0001), race (black: HR 1.22, 95% CI 0.81–1.83; P = 0.3356), site (lower: HR 1.57, 95% CI 1.12–2.21, P < 0.0001) and stage (IB: HR 3.17, 95% CI 1.68–6.01, P = 0.0004; IIA: HR 3.98, 95% CI 2.03–7.82, P = 0.0001; IIB: HR 8.11, 95% CI 4.28–15.37, P < 0.0001; IIC: HR 9.53, 95% CI 4.93–18.42, P < 0.0001; IIIA: HR 13.40, 95% CI 6.88–26.08, P < 0.0001; IIIB: HR 15.91, 95% CI 8.63–29.35, P < 0.0001 and IIIC: HR 31.74, 95% CI 16.98–59.36, P < 0.0001) were included into the Cox model (stepwise model) for CSS. The results of the univariate and multivariate analyses are presented in Tables 2 and 3.
Since SEER data did not contain detailed information on treatments upon recurrence and adjuvant therapy, treatments were not incorporated into the nomogram. To assess the impact of study years on survival, we analyzed patient survival from two time periods (2004–2008 vs. 2011–2015) using Kaplan–Meier curves (Supplementary Fig. 1) and found that the survival of patients in the later period was better than that in the earlier period (prior to the widespread adoption of many effective adjuvant therapies) but the difference was not statistically significant (p = 0.29).
3.3 Prognostic nomogram for predicting OS and CSS
The prognostic nomogram to predict the 2-, 4-, and 6-year OS (Fig. 2A) and CSS (Fig. 2B) were constructed based on the above risk factors. By drawing a straight line upward to the points axis, the specific score of each corresponding independent risk factor can be obtained. The total points present the sum of the score of each factor. The corresponding predicted probability of the 2-, 4- and 6-year OS and CSS can be achieved by drawing a straight line down from the total points axis to the survival probability axes. Taking the nomogram of OS as an example, a 60-year-old (51 points) Asian (0 points) male (23 points) patient with stage IIB (56 points) ALM has a sum-point of OS equal to 130, corresponding to a predicted 2-, 4- and 6-year OS of 81%, 62%, and 48%, respectively.
Prognostic nomogram of ALM patients' 2-, 4- and 6-year OS (A) and CSS (B). By drawing a straight line upward to the points axis, the specific score of each corresponding independent risk factor can be obtained. The total points present the sum of the score of each factor. The corresponding predicted probability of the 2-, 4- and 6-year OS or CSS can be achieved by drawing a straight line down from the total points axis to the survival probability axes. Age measurement unit: year
3.4 Calibration and discrimination of the nomogram
The calibration curves show a favorable consistency between the nomogram predictions and the actual observed outcomes of the 2-, 4- and 6-year OS and CSS in the training and internal validation cohorts (Fig. 3). As indicated by the C-index values, the nomogram of OS presents good prognostic accuracy and clinical applicability (training cohort: 0.727; internal validation cohort: 0.722; external validation cohort: 0.682), which correspond to the AUC values in the ROC curve analysis (0.75, 0.77 and 0.78 in the training cohort; 0.79, 0.76 and 0.75 in the internal validation cohort at 2, 4 and 6 years respectively). For the nomogram of CSS, it achieved a favorable efficacy with a C- index of 0.791 and 0.774 in the training and internal validation cohort, respectively. The AUC of 2-,4-, and 6-year CSS were 0.84, 0.83, and 0.84 in the training set, and 0.84, 0.82, and 0.80 in the internal validation set, respectively (Fig. 4).
Calibration curves predicting the 2-, 4- and 6-year OS and CSS of patients in the training cohort (A, C), and internal validation cohort (B, D). The x-axis indicates the predicted survival probability and the y-axis indicates the actual survival probability at 2, 4, or 6 years. The blue line indicates that the prediction agrees with actuality. OS, overall survival; CSS: cancer-specific survival
4 Performance of the nomogram in external validation cohorts
The nomogram of OS was then externally validated in a multicenter database. The C-index of the external validation set was 0.657. The ROC curves for predicting the 2-, 4-, and 6-year OS of stage I-III ALM patients are shown in Fig. 5A, with the AUCs of 0.67, 0.72, and 0.65, indicating that the nomogram has good discrimination. In addition, the calibration curve of the external validation cohort was shown in Fig. 5B, suggesting good consistency between the predicted situation and the actual survival of patients.
5 Discussion
ALM is found on peripheral body parts such as soles, palms, and nail beds. The incidence of melanoma is highest in white Caucasian populations, while ALM accounts for less than 10% of the cases and is one of the least common cutaneous melanoma histological subtypes [17]. Nevertheless, in populations with darker and more heavily pigmented skin where melanoma incidence rates are lower, ALM takes up to 50% of all cases with melanoma [17]. Since the etiology of ALM is largely unrelated to intermittent sun exposure, the actual incidence rates of ALM are similar for all ethnic groups [18, 19]. To date, the specific pathogenesis of ALM remains largely unknown, but mechanical injury or stress to the site may play an important role [20, 21]. Whole-genome sequencing studies have shown that ALM development is driven by structural changes and mutation signatures of unknown etiology [19].
Compared with other histological subtypes of cutaneous melanoma, ALM is more aggressive and mainly detected at a relatively advanced stage with a worse prognosis [17, 20], mainly due to late diagnosis, genetic features as well as socioeconomic factors [22,1), consistent with nomogram findings. In addition, determining the exact cause of death can be challenging, particularly in retrospective studies. Our external validation set lacks CSS data and therefore cannot validate the nomogram for CSS. Additional validation on larger and more comprehensive datasets is warranted. Finally, due to the small sample size of the external validation cohort, it is imperative to obtain additional validation cohorts with a larger sample size for the nomogram. A future model based on a larger sample size of multicenter data incorporating other clinical information progressively in the future may facilitate the prognostic value of this model.
6 Conclusions
We developed and validated nomograms to provide convenient and reliable tools for prognosis prediction for ALM patients. More future prospective studies are warranted to confirm this result.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the SEER database repository, https://seer.cancer.gov/. The external validation cohort used and analyzed during the current study is available from the corresponding author upon reasonable request.
Abbreviations
- OS:
-
Overall survival
- CSS:
-
Cancer-specific survival
- ALM:
-
Acral lentiginous melanoma
- SEER:
-
Surveillance, Epidemiology, and End Results
- HR:
-
Hazard ratio
- TNM:
-
Tumor, Node, Metastasis
- ICD‐O:
-
International Classification of Disease for Oncology
- AJCC:
-
American Joint Committee on Cancer
- FUSCC:
-
Fudan University Shanghai Cancer Center
- TMUCIH:
-
Tian** Medical University Cancer Institute and Hospital
- ZJCH:
-
Zhejiang Cancer Hospital
- CI:
-
Confidence interval
- C-index:
-
Concordance index
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49.
Weiss SA, Wolchok JD, Sznol M. Immunotherapy of Melanoma: Facts and Hopes. Clin Cancer Res. 2019;25(17):5191–201.
Landow SM, Gjelsvik A, Weinstock MA. Mortality burden and prognosis of thin melanomas overall and by subcategory of thickness, SEER registry data, 1992-2013. J Am Acad Dermatol. 2017;76(2):258–63.
Garbe C, Keim U, Amaral T, et al. Prognosis of Patients With Primary Melanoma Stage I and II According to American Joint Committee on Cancer Version 8 Validated in Two Independent Cohorts: Implications for Adjuvant Treatment. J Clin Oncol. 2022;40(32):3741–9.
Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718–29.
Rogers GS, Kopf AW, Rigel DS, et al. Influence of anatomic location on prognosis of malignant melanoma: attempt to verify the BANS model. J Am Acad Dermatol. 1986;15(2 Pt 1):231–7.
Garbe C, Büttner P, Bertz J, et al. Primary cutaneous melanoma. Prognostic classification of anatomic location. Cancer. 1995;75(10):2492–8.
Gillgren P, Brattström G, Frisell J, et al. Effect of primary site on prognosis in patients with cutaneous malignant melanoma. A study using a new model to analyse anatomical locations. Melanoma Res. 2005;15(2):125–32.
Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186(5):1342–8.
Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Arch Dermatol. 2009;145(4):427–34.
Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30(31):3834–40.
Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70.
Valentini V, van Stiphout RGPM, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29(23):3163–72.
Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180.
Shariat SF, Karakiewicz PI, Suardi N, et al. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14(14):4400–7.
Wang Y, Li J, **a Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–95.
Desai A, Ugorji R, Khachemoune A. Acral melanoma foot lesions. Part 1: epidemiology, aetiology, and molecular pathology. Clin Exp Dermatol. 2017;42(8):845–8.
Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18(8):775–84.
Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–80.
Durbec F, Martin L, Derancourt C, et al. Melanoma of the hand and foot: epidemiological, prognostic and genetic features. A systematic review. Br J Dermatol. 2012;166(4):727–39.
Minagawa A, Omodaka T, Okuyama R. Melanomas and Mechanical Stress Points on the Plantar Surface of the Foot. N Engl J Med. 2016;374(24):2404–6.
Teramoto Y, Keim U, Gesierich A, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol. 2018;178(2):443–51.
Howard MD, **e C, Wee E, et al. Acral lentiginous melanoma: differences in survival compared with other subtypes. Br J Dermatol. 2020;182(4):1056–7.
Carrera C, Puig-Butille JA. Clinical, Epidemiological, and Molecular Heterogeneity in Acral Melanoma. J Invest Dermatol. 2018;138(2):254–5.
Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20–33.
Zhou L, Shao L, Gao S, et al. Impact of response patterns for patients with advanced acral melanoma treated with anti-programmed death-1 monotherapy. Br J Dermatol. 2023;188(1):112–21.
Cui C, Wang X, Lian B, et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. J Immunother Cancer. 2022;10(4):e004307.
Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–92.
Park YH, Senkus-Konefka E, Im SA, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31(4):451–69.
Guo Q, Wu M, Li H, et al. Development and validation of a prognostic nomogram for myocardial infarction patients in intensive care units: a retrospective cohort study. BMJ Open. 2020;10(12):e040291.
Yin T, Zhao Y, Yang Y, et al. Nomogram for Predicting Overall Survival in Acral Lentiginous Melanoma: A Population-based Study. Int J Gen Med. 2021;14:9841–51.
Yang J, Pan Z, Zhao F, et al. A nomogram for predicting survival in patients with nodular melanoma: A population-based study. Medicine (Baltimore). 2019;98(24):e16059.
Wang S, Chen Y, Sun J, et al. Development and validation of two online dynamic nomograms for patients with non-distant metastatic cutaneous melanoma based on surgical approaches. Cancer Med. 2023;12(18):18479–90.
Yang C, Liao F, Cao L. Web-based nomograms for predicting the prognosis of adolescent and young adult skin melanoma, a large population-based real-world analysis. Transl Cancer Res. 2020;9(11):7103–12.
Yue H, Xu B, Gao J, et al. A Novel and Easy-to-Promote Prognostic Model for Patients With Uveal Melanoma. Front Oncol. 2022;12:879394.
Huang JN, Yu H, Wan Y, et al. A prognostic nomogram for the cancer-specific survival of white patients with invasive melanoma at BANS sites based on the Surveillance, Epidemiology, and End Results database. Front Med (Lausanne). 2023;10:1167742.
Varey AHR, Li I, El Sharouni MA, et al. Predicting Recurrence-Free and Overall Survival for Patients With Stage II Melanoma: The MIA Calculator. J Clin Oncol. 2024;42(10):1169–80.
Balch CM, Soong SJ, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20(12):3961–8.
Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65(5 Suppl 1):S78–86.
Enninga EAL, Moser JC, Weaver AL, et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017;6(10):2203–12.
Behbahani S, Malerba S, Samie FH. Acral lentiginous melanoma: clinicopathological characteristics and survival outcomes in the US National Cancer Database 2004–2016. Br J Dermatol. 2020;183(5):952–4.
Lichte V, Breuninger H, Metzler G, et al. Acral lentiginous melanoma: conventional histology vs. three-dimensional histology. Br J Dermatol. 2009;160(3):591–9.
Howard M, **e C, Wee E, et al. Acral lentiginous melanoma: Clinicopathologic and survival differences according to tumour location. Australas J Dermatol. 2020;61(4):312–7.
Asgari MM, Shen L, Sokil MM, et al. Prognostic factors and survival in acral lentiginous melanoma. Br J Dermatol. 2017;177(2):428–35.
Acknowledgements
This work was supported by the Shanghai Science and Technology Development Funds (19411951700 to Y. Chen), LinGang laboratory “Seeking Distinguished Young Scholars” project (LG-QS-202205-11 to W. Sun), and the National Natural Science Foundation of China (81802636 to W. Sun). The funders did not play a role in the manuscript design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
TL and YC designed the study. TL, YL, WS, TL, and JY did the data acquisition and analysis. YX, CW, and WY performed the statistical analysis. TL, WS, and YC wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board and Ethics Committee of the Fudan University Shanghai Cancer Center, Tian** Medical University Cancer Institute and Hospital, and Zhejiang Cancer Hospital. As this study used anonymous data relating to patients, all the institutes mentioned above waived the requirement for signed informed consent. All procedures in our study were to the corresponding guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
44178_2024_92_MOESM1_ESM.pdf
Supplementary Material 1: Supplementary Figure 1. Differences in overall survival (OS) of two time periods (2004-2008 vs. 2011-2015).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, T., Sun, W., Luo, Y. et al. A novel survival nomogram for stage I-III acral lentiginous melanoma patients, based on the SEER database and a multi-center external validation cohort. Holist Integ Oncol 3, 23 (2024). https://doi.org/10.1007/s44178-024-00092-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00092-x