Abstract
Lithium-sulfur batteries (LSBs) have garnered significant attention as a promising next-generation rechargeable battery, offering superior energy density and cost-effectiveness. However, the commercialization of LSBs faces several challenges, including the ionic/electronic insulating nature of the active materials, lithium polysulfide (LiPS) shuttle effect, volume expansion/contraction of the cathode, and issues with Li metal anode. Despite numerous efforts to address these challenges, previous studies have predominantly been conducted under mild conditions such as high electrolyte-to-sulfur (E/S) ratio, low sulfur loading, and excess Li metal, which cover issues related to Li metal anode. However, for realizing high-energy–density LSBs, practical conditions such as low E/S ratio, high sulfur loading, and limited Li metal are essential. Under these conditions, the increased current on Li metal and higher LiPS concentration exacerbate issues with Li metal anode such as dendrite growth, dead Li, high reactivity with electrolyte, and high reactivity with LiPSs. These problems lead to rapid failure of Li metal, significantly impacting the electrochemical performance of LSBs. Consequently, protecting Li metal anode is crucial for the practical LSBs. This paper introduces the challenges associated with Li metal anode in LSBs and reviews research focused on protecting Li metal anode in each battery component: anode, electrolyte, cathode, and separator/interlayer. Finally, we discuss future research directions of each component towards practical LSBs.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The depletion of fossil fuels and the need for carbon neutrality has significantly intensified the interest in sustainable electrical energy conversion and storage devices [1, 2]. Over the past three decades, Li-ion batteries (LIBs) based on intercalated composite cathodes and anodes have been widely used as rechargeable batteries, thus dominating the battery market [3, 4]. However, the theoretical capacity of cathode materials such as LiCoO2 and LiFePO4 used in LIBs is around 200 mAh g−1, and they are currently approaching their limits [5]. Consequently, with the expanding market for electric vehicles (EVs) and energy storage systems (ESSs), there has been a recent surge in research interest in high-energy–density next-generation rechargeable batteries [6,7,8,9,10,11,12]. Lithium-sulfur batteries (LSBs) utilize sulfur as the cathode material, exhibiting a specific capacity of 1675 mAh g−1, and Li metal as the anode material with an ultrahigh theoretical capacity of 3860 mAh g−1, which makes LSBs a promising candidate for achieving an energy density of LSB cells above 500 Wh kg−1 [13,38,39,40]. (2) A high concentration of LiPSs (> 6.0 M [S] species) in the electrolyte driven by high sulfur loading and low E/S ratio induces severe shuttle effects, promoting side reactions between Li and LiPSs [41, 42]. (3) With a low negative-to-positive electrode ratio (N/P ratio < 2) and E/S ratio, limited amounts of Li and the electrolyte cannot withstand continuous side reactions, leading to rapid cell failure [43, 44]. These issues, coupled with the intrinsic challenges of Li metal, such as dendrite growth and the formation of dead Li, synergistically contribute to the rapid capacity decay and premature cell failure [45, 46].
Consequently, to realize high-energy–density LSBs, it is necessary to focus on protecting Li metal. This paper discusses the issues related to Li metal in LSBs and reviews various strategies for protecting Li metal across battery components: anodes, electrolytes, cathodes, and separators/interlayers. Finally, we present research directions for each component to realize practical LSBs.
2 Electrochemistry of LSBs
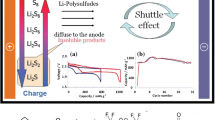
Typical LSBs consist of an elemental sulfur cathode, a Li metal anode, a separator, and an ether-based electrolyte (primarily 1 M lithium bis (trifluoromethanesulfonyl) imide (LiTFSI) in DOL (1,3-dioxolane) / DME (1,2-dimethoxyethane) (1:1, by volume) with 2 wt. % LiNO3), and operate based on the conversion reactions between sulfur and Li (Fig. 1a) [47]. During discharging, elemental sulfur reacts with Li+ to form lithium sulfide (Li2S), which decomposes back into Li and sulfur during charging (Eq. (1)). This reaction mechanism, which involves the participation of 16 electrons, endows LSBs with a theoretical capacity of 1675 mAh g−1, significantly exceeding the capacity of cathode materials used in LIBs by over five times [48, 49].
In LSBs, the electrochemical processes are considerably complex. During the discharge process, elemental sulfur transforms into a long-chain LiPS intermediate (Li2Sx, 4 ≤ x ≤ 8) which is soluble in ether-based solvents and is eventually converted into lithium sulfides (Li2S2/Li2S), following a “solid–liquid-solid” conversion mechanism [5]. The transition from sulfur to various sulfur compounds, including insoluble lithium sulfides and soluble LiPSs, plays a critical role in defining the electrochemical characteristics of LSBs [50]. The discharge mechanism of the LSBs can be categorized into four distinct stages (Fig. 1b).
In stage I, solid sulfur reacts with Li and electrons from the Li metal anode to form soluble long-chain Li2S8 via a solid–liquid reaction (Eq. (2)). This reaction occurs at a relatively high voltage of approximately 2.3 V, and is characterized by a slightly slo** plateau. Here, elemental sulfur in the cathode dissolves in Li2S8, causing the cathode to become porous, leading to volumetric contraction [51].
The stage II involves the continuous reduction of Li2S8 to a liquid-state Li2S4 (Eqs. (3) and (4)), which constitute a “liquid–liquid” reaction. This stage leads to an increase in the electrolyte viscosity owing to the increasing concentration of LiPSs in the electrolyte, resulting in a voltage peak at the end of the second stage owing to an increased overpotential [52]. Stages I and II collectively contributed one-fourth (419 mAh g−1) of the total theoretical capacity of the LSBs.
In the stage III, liquid Li2S4 is reduced to solid-state lithium sulfides (Li2S2/Li2S), following a “liquid–solid” reaction (Eqs. (5) and (6)). This process forms a long second plateau in the discharge curve, occurring around 2.1 V.
Stage IV involves the transformation of residual Li2S2 into the final product, Li2S, which is characterized by a “solid–solid” reaction (Eq. (7)). Consequently, this stage is associated with high overpotential and slow kinetics, leading to a rapid voltage drop [53]. Stages III and IV contributed three-fourths (1256 mAh g−1) to the total capacity.
During charging, the reverse reaction of the discharge process occurs: solid-state Li2S is oxidized, going through intermediate soluble LiPSs, and ultimately converting back to S8. The small voltage peak observed in the initial part of charging was attributed to the overpotential occurring during the decomposition of Li2S [54].
3 Challenges of Li metal anode in LSBs
The use of Li metal anodes presents various inherent challenges, including Li dendrite formation, dead Li, uneven surface morphology, electrolyte depletion, and gassing problems. These issues become more pronounced when LiPSs dissolve in the electrolyte in LSBs, thereby diminishing the cycle stability and safety (Fig. 2a). Furthermore, as discussed previously, Li degradation occurs more rapidly and severely in LSBs under practical conditions. This section introduces the problems associated with Li metal in LSBs and categorizes them into three main types: Li dendrites & dead Li, high reactivity with electrolyte & LiPSs.
a Schematic illustration of challenges of Li metal anode in LSBs. b Schematic diagram of Li dendrite strip** models: tip-strip** model, base-strip** model, and tip-/base-strip** model (Reprinted with permission from [63], 2021, John Wiley and Sons)
3.1 Li dendrite and dead Li
The formation of Li dendrites and dead Li is the most severe challenge to the stability and safety of Li metal anode [55,56,57]. Li dendrites are thin branching structures that can form on the surface of anodes, causing a low CE, capacity decay, and short circuits. These dendrites pose several challenges for the operation of LSBs. First, compared with Li with flat surfaces, Li dendrites have greater specific surface areas, inducing undesirable reactions at the surface. These side reactions consume electrolytes and metallic Li, resulting in poor cycle life of LSBs. Second, repeated side reactions can destroy the dendritic structure, causing Li to lose contact with the anode. When Li+ lose contact, they are unable to participate in the electrochemical reaction, becoming “dead Li” which cannot return to the anode [55]. Finally, the Li dendrites can penetrate the polymer separator and reach the cathode, causing safety hazards in short circuits [58]. More dangerously, short circuits typically happen with battery thermal runaway, causing spontaneous combustion and explosions.
Under electrodeposition conditions, dendrite growth is an unavoidable phenomenon for metal anodes such as Li, Zn, Cu, Ag, and other metals due to their 'host-less' property, unlike graphite anode. The “Space Charge Model” theory, proposed by Chazalviel in 1990, revealed how the rapid depletion of ions near the electrode surface causes space charge and electric field to arise in the electrode/electrolyte interface, accelerating the deposit of massive Li+ [59]. This phenomenon is related to the gradient in the Li+ concentration between the two electrodes during the electroplating process, which may be increased by the applied current, as shown in Eq. (8).
where J referred to the current density, e is the elementary charge, D is the ion diffusion coefficient, \({\upmu }_{{\text{a}}}\) and \({\upmu }_{{{\text{Li}}}^{+}}\) are the mobility of anion and Li+, respectively.
The ionic concentration gradients remained constant when the concentration gradient was less than 2C0/L, resulting in smooth deposition. However, when the gradient exceeded 2C0/L, the formation of a local space charge eventually destroyed the potential balance on the electrode surface after a certain period. This period is the “Sands Time, τ” [60]:
where C0 is the initial concentration of Li salts, L is the distance between the electrodes, and ta is the transference number of anions. In LSBs, where high current is applied to the Li metal anode due to the high capacity of the cathode active material, the Sands time decreases according to Eq. (9), which leads to an environment that exacerbates dendrite formation.
The Li dendrites formed by this mechanism can break during cycling and form dead Li. Dead Li is defined as Li that has lost contact with the electrode and is therefore unable to participate in electrochemical reaction [56, 61, 62]. This hindered the transport of Li and decreased the CE, resulting in a poor cycle life. Dead Li is formed not only by broken dendrites but also by uneven current distribution during the strip** process. As LSB are systems that start from the strip** process, unlike LIB, it is crucial to prevent the formation of dead Li during discharge. Figure 2b shows the formation of dead Li during the strip** [63]. The Li metal is electrochemically oxidized to Li+ during strip**, which departs from the anode and moves through the solid electrolyte interphase (SEI) layer to the bulk electrolyte. Thus, when controlling the strip** process, it is crucial to consider the rates of Li+ diffusion inside the anode, ionic diffusion through the SEI layer, and reaction at the SEI-electrolyte interface. However, in LSBs, LiPSs are rapidly reduced at the anode to form an SEI layer composed of components with low Li+ conductivity, such as Li2S2/Li2S (1.90 × 10–26 S cm−1), which makes uniform strip** challenging. During the strip** process, strip** occurs locally at defect sites within the insulating layer, resulting in the formation of pits on the Li surface. In subsequent plating, electric charges are higher in areas of high curvature, leading to Li nucleation and growth within the pit mouth [64, 65]. This mechanism results in a porous Li structure. Several studies have reported that this pitting mechanism significantly induces the formation of dead Li and Li dendrite growth [66, 67].
As shown in Fig. 2b, three dissolving sites–tip, base, and combined tip/base–were used to discuss the strip** electrochemistry. In the tip-strip** model, Li dendrites maintain good contact with the substrate during the strip** process, preventing the formation of dead Li. This is ideal for dendritic strip**. However, the base-strip** model is a typical phenomenon because greater curvature at the dendrite neck tends to accumulate more electron densities, leading to faster rates of Li dissolution. After dendritic breakage, the electrolyte corrodes the newly exposed Li surface, resulting in the accumulation of a SEI layer with low electrical conductivity around the broken Li. Consequently, Li is no longer electrically connected to the anode and cannot participate in the electrochemical reaction. Additionally, based on the tip-/base-strip** model, the dendritic tip and base are considered to be the active areas for dendritic dissolution, which also results in dead Li.
3.2 High reactivity with electrolyte and LiPSs
The ultralow reduction potential (-3.04 V vs. standard hydrogen electrode (SHE)) of the Li metal anode makes it highly reactive with electrolyte. In 1971, Dey made the initial study of the thin film formed through the reaction of electrolyte and Li [68], and Peled described it as “SEI” in 1979 [69]. Goodenough et al. clarified the link between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of electrolytes and SEI production on electrodes [70]. The chemical potential of Li metal is well above the LUMO of practical organic electrolyte solvent molecules and anions. Within milliseconds or less, a significant reduction process between the free electrons in Li and the electrolyte begins when bare Li is exposed to the electrolyte. This severe parasitic reaction limits the CE of Li strip**/plating cycles by causing an irreversible loss of Li and the electrolyte. Moreover, the decomposition of DOL and DME generates gases such as CH4 and H2. Gas evolution causes the pouch cell to expand, resulting in safety issues [71, 72]. In conclusion, it is essential to suppress the reactivity of Li to improve cycle life by inducing a robust SEI layer.
Calendar aging due to corrosion also occurs because of the high reactivity of Li metal, which has also recently been reported to cause loss of Li metal and shorten its cycle life [73,74,75]. In the Li metal anode, unlike the graphite anode, each electrodeposition cycle exposes fresh Li metal surfaces. Consequently, the SEI layer was reformed at each cycle so that the potential of the Li metal was 0 V vs. Li/Li+ in the open circuit. This makes calendar aging of the Li metal anode critical. It has been reported that Li metal usually experiences a loss of approximately 2–3% of its capacity after 24 h of calendar aging [75]. Especially in an environment with a limited amount of electrolyte and Li at the practical level of the pouch cell, it is critical to suppress calendar aging to improve stability.
In LSBs, the LiPS intermediates are soluble in ether-based electrolytes. During battery operation, high-order LiPSs dissolve from the cathode and diffuse toward the anode owing to concentration gradients [76]. Owing to the strong reductive capability of Li metal and the oxidative nature of LiPSs, spontaneous reduction of LiPSs occurs at the anode, forming soluble low-order LiPSs or insoluble Li2S2/Li2S which are deposited on the Li metal [77]. The produced low-order LiPSs subsequently diffuse back to the cathode, a process known as the “LiPS shuttle effect” [78]. The phenomenon of soluble LiPSs migrating between the sulfur cathode and Li metal anode leads to substantial degradation of the Li metal anode through severe corrosion, as well as a notable reduction in the active materials, making “dead sulfur” [79]. In an electrolyte composed of DOL, DME, and LiTFSI, LiPSs preferentially react with Li compared to the other components, as evidenced by the calculation of the Gibbs free energy change [80]. This led to the formation of a Li2S2/Li2S-rich SEI layer on the Li surface. This phenomenon is more pronounced under practical conditions of high sulfur loading and low E/S ratios, where the concentration of LiPSs in the electrolyte increases dramatically.
However, Li2S2/Li2S, with their low ionic conductivity (1.90 × 10–26 S cm−1), are inadequate as SEI layer components, increasing interfacial resistance due to their inability to facilitate Li+ transfer [81]. Therefore, Li strip** predominantly occurs at the defect sites of the insulating layers, forming uneven and discrete pits, a situation exacerbated under a high current density driven by high sulfur loading [82]. During the subsequent charging process, LiPSs react with Li dendrites to form mossy Li [83]. The increased surface area of this morphology leads to the formation of a thin SEI layer, allowing easy penetration of LiPSs and reaction with the newly plated Li [36]. In summary, the presence of LiPSs in the electrolyte intensifies the issues of dendrite growth, dead Li, electrolyte decomposition, and gas evolution, as discussed in Sects. 3. 1 and 3. 2. This contributes to the formation of a new Li2S2/Li2S layer, perpetuating a vicious cycle, and ultimately leading to the rapid failure of the Li metal. Moreover, the reaction of LiPSs at the anode contributes to self-discharge, leading to a reduction in the open-circuit voltage (OCV) and capacity decay [84].
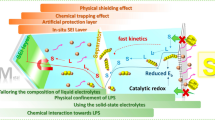
To summarize, in LSBs, high current density and the formation of Li2S2/Li2S-rich SEI layer from the reduction of LiPSs on the Li metal anode lead to severely uneven Li strip**/plating. Consequently, substantial Li dendrite growth and the formation of dead Li occur at the anode. Additionally, in LSBs where Li strip** occurs first, the Li structure becomes porous. This leads to a mossy Li surface, continuously exposing new surfaces that react with the electrolyte and LiPSs, resulting in gassing problems, depletion of electrolyte and Li, continuous loss of active material, and further uneven Li growth again. These phenomena are especially pronounced under the practical conditions of LSBs, characterized by low E/S and N/P ratios, ultimately accelerating cell failure. Therefore, protecting the Li metal is essential for the commercialization of LSBs. Strategies for protecting the Li metal include suppressing the LiPS shuttle effect and directly stabilizing it [85,86,87]. In the following section, we review the various strategies employed for each part (anode, electrolyte, cathode, and separator/interlayer) of the battery and present research directions for each component toward achieving LSBs with high-energy–density and stability.
4 Li metal anode engineering
The most direct approach for addressing anode-related issues in LSBs is to engineer Li metal anode [88,89,90,91]. Previous studies have primarily focused on two strategies: (1) introducing an artificial SEI layer and (2) incorporating a host material for Li. These methodologies aim to proactively tailor Li metal anodes, mitigate dendrite formation, and minimize their reactivity with LiPSs. By implementing these strategies, advancements can be made toward achieving enhanced the LSB performance. In addition, studies are currently being conducted to implement a scalable process while maintaining thin Li metal anodes for practical LSB.
4.1 Artificial SEI layer
Introducing an artificial solid electrolyte interface (SEI) layer into LSBs is important for enhancing their stability and performance. This SEI layer serves as a pivotal factor influencing the electrochemical kinetics of the Li metal anode, ensuring uniformity in the process of Li plating and strip** during cycling. Its versatile functions include preventing undesirable reactions between the anodes and electrolytes, facilitating uniform Li deposition, preventing dendrite formation, and improving the overall cycle life. An optimal artificial SEI layer requires attributes such as high Li+ conductivity, robust mechanical properties, resistance against corrosion by LiPSs, and scalability [92,93,94]. To meet these requirements, researchers have explored a spectrum of materials with organic, inorganic, and composite compositions. An organic SEI layer is generally flexible and can adapt to anode volume changes, whereas an inorganic SEI layer is mechanically rigid and can improve surface stability. By combining inorganic and organic components in an appropriate structure, the synergistic effect of different components can achieve highly stable and safe Li metal anodes [95, 3d). The symmetric LiPON-coated Li metal cells demonstrated durable and non-dendritic cycling for over 900 cycles at a current density of 3 mA cm−2. Furthermore, a Li–S pouch cell using LiPON-coated Li was obtained with a specific energy density of approximately 300 Wh kg−1, maintaining a relatively stable CE of around 91%. It exhibited an extended cycle life, retaining over 1.0 Ah capacity of over 120 cycles (Fig. 3e). Luo et al. presented a scalable method for fabricating a tuned Li anode with an in-situ formed LiF-dominant coating, marking the first successful application of such a method in LSBs [105]. Ammonium fluoride (NH4F) was used as a fluorine source for a LiF dominant artificial SEI layer. This layer acts as a mechanically and chemically stable, ionically conductive interface layer on the Li metal surfaces. In LSBs using tuned Li, it was confirmed through the scanning electron microscopy (SEM) images that corrosion and interfacial degradation of Li anode were significantly reduced. The coating serves as a chemically stable interfacial layer that prevents attack from the electrolyte/LiPSs and it also functions as an ionic conductor that facilitates smooth Li+ flux and regulates uniform Li deposition (Fig. 3f). Consequently, a Li–S pouch cell with a high-loading sulfur and tuned Li anode maintained a capacity of 680 mAh g−1 after 45 cycles, with a high discharge capacity of 136 mAh (Fig. 3g).
4.1.3 Composite artificial SEI layer
Composite artificial SEI layers are considered a significant advancement in improving the performance and stability of Li-based battery technologies, especially for LSBs and Li metal batteries [116].
(2) The inactive component within the Li-containing alloy hosts, featuring an interconnected continuous 3D network, maintained compositional stability and structural integrity even under considerable volume changes, thereby reducing the local current density.
(3) Li-containing alloys exhibit lower reactivity than Li metal because other materials in the alloy structure limit the chemical reaction of Li metal, preventing side reactions of Li with the electrolytes. This characteristic also enhances the air stability of Li metal anodes against oxidation and moisture [117].
(4) The preparation of Li alloy hosts involves a straightforward and simple process under moderate conditions, which is conducive to the large-scale application of Li metal anodes.
Kong et al. proposed a Li-rich lithium-magnesium (Li-Mg) alloy as a promising anode for LSBs [118]. The alloy developed a porous, interconnected structure through electrochemical dealloying. The presence of Mg in the SEI layer enhances its stability, thereby preserving a smooth surface morphology. After Li strip**, a conductive Li-poor Li-Mg alloy matrix emerged, exhibiting high electric and ionic conductivities, effectively serving as both an excellent current collector and a host for subsequent Li plating (Fig. 5a). This conductive Li-Mg alloy matrix contributes to the maintenance of the microstructural and bulk integrity during cycling. In addition, the surface morphologies and X-ray photoelectron spectroscopies (XPS) of the cycled Li-Mg alloys were significantly different from Li metal, suggesting that the Li-Mg alloys have higher resistance to electrolyte corrosion. As a result, LSBs featuring Li-Mg alloy anodes retained a discharge capacity of 606.5 mAh g−1 after 200 cycles, surpassing those employing Li metal anodes, which delivered 433.6 mAh g−1 at 0.1 C (Fig. 5b). ** and plating, culminating in impressive rate capability (650 mAh g−1 at 5 C) and extended cycle longevity exceeding 600 cycles, displaying a minimal capacity decay of only 0.051% per cycle within LSBs. In a configuration comprising S/TiN-VN@CNFs||Li/TiN-VN@CNFs, the LSBs exhibited a notable areal capacity of 5.5 mAh cm−2 even when accommodating a sulfur loading of 5.6 mg cm−2. Furthermore, the good mechanical flexibility of the working electrodes is demonstrated by the pouch cell tested under LED illumination.
Wei et al. introduced a conductive composite architecture comprising bio-derived N-doped porous carbon fiber bundles (N-PCFs) hosting cobalt and niobium carbide nanoparticles (NbC/Co ⊂ N-PCFs) as an integrated multifunctional platform designed to address challenges in both Li metal anode and sulfur cathode (Fig. 6b) [123]. This composite, which features synergistically enhanced electrical conductivity and surface polarization, incorporates highly conductive NbC and Co within a hierarchical porous N-PCF matrix. When employed as a host for Li-metal anodes, the composite displayed robust lithiophilicity and facilitated favorable adsorption, diffusion, and uniform Li deposition, resulting in a nondendritic morphology. Utilized as the host for the sulfur cathode, this conductive, porous, and polar architecture endows the NbC/Co ⊂ N-PCFs with a combined functionality involving three-dimensional physical confinement, robust chemical trap**, and electrocatalytic effects, fostering a high sulfur content of 78% and expediting the kinetics of conversion reaction. The LSBs with S@NbC/Co ⊂ N-PCFs and NbC/Co ⊂ N-PCFs@Li demonstrate remarkable stability in cycling performance, exhibiting a capacity retention of approximately 82.3% after 500 cycles at 0.5 C, while accommodating a sulfur loading of 3.4 mg cm−2.
5 Electrolyte engineering
Organic liquid electrolytes, particularly those based on ethers, are widely employed in LSBs owing to their notable attributes, such as high ionic conductivity, favorable interfacial contact with electrodes, and minimal side reactions with Li. Acceptable solvents for LSB electrolytes are predominantly restricted to linear and cyclic ethers such as DME and DOL because other common electrolyte solvents such as esters, carbonates, and phosphates tend to react with LiPSs. In this context, linear DME exhibits heightened solubility and quicker reaction kinetics for LiPSs, but with greater reactivity toward Li metal. Conversely, cyclic DOL creates a more robust solid-electrolyte interface on the Li surface. Hence, combining DME and DOL results in synergistic effects on both the specific capacity and retention of sulfur, surpassing the performance of either solvent used individually [52, 124, 125]. However, the dissolution of intermediate LiPSs poses significant challenges, necessitating the incorporation of appropriate additives to safeguard the Li metal anodes or modify the structure of Li+ ions. Electrolyte engineering has emerged as a crucial approach for regulating the SEI layer, curbing dendrite formation on Li-metal anodes, and managing the interactions between LiPSs and Li metal. This section describes electrolyte engineering employing two key strategies: (1) manipulation of the SEI layer constituents and dendrite suppression via additive integration, and (2) diminishing the solubility of LiPSs within the electrolyte to mitigate its interaction with the anode.
5.1 Additives
Additives, typically comprising less than 5 to 10% by weight or volume, are incorporated into the electrolyte to enhance the cycling performance of cells. These components play an active role in SEI formation, effectively curbing the reactivity of the Li metal anode and mitigating the LiPS shuttling effect. Among these additives, LiNO3 has received considerable attention for LSBs because of its ability to safeguard Li metal anodes by fostering the creation of a robust nitrogen-based SEI layer [126, 127]. Many additives are actively under investigation beyond LiNO3, investigated to facilitate SEI formation and dendrite inhibition.
Li et al. introduced a straightforward and efficient approach to hinder the growth of Li dendrites on Li metal anodes by employing thionyl chloride (SOCl2) as an electrolyte additive, facilitating the in situ formation of a stable interfacial protective layer containing LiCl and Li2SO3 [128]. This dense SEI layer established on the Li metal anode effectively curtailed the shuttling of LiPSs during cycling and also inhibited Li corrosion. Additionally, when the Li metal anode is shielded from the electrolyte by the LiCl-enriched surface film, SOCl2 decomposes to generate active sulfur. This sulfur acts as a 'redox additive,' providing additional capacity to the cathode in the LSBs (Fig. 7a). Consequently, employing 2% SOCl2 yielded LSBs showcasing notably high discharge capacity (2202.3 mAh g−1 at 400 mA g−1) and exceptional rate performance (1348.6 mAh g−1 at 3000 mA g−1), exhibiting significant cycling stability (Fig. 7b). Lian et al. introduced a novel multifunctional electrolyte additive, 1,4-benzenedithiols (BDT), for LSBs [129]. The thiol group within 1,4-BDT underwent oligomerization with sulfur, forming S–S bonds that modified the original sulfur redox pathway and impeded the shuttling of LiPSs. The formation of S–S bonds between thiol groups and sulfur offers several benefits for LSBs. These include stabilizing sulfur sites, activating dead sulfur atoms, improving electrochemical kinetics, and facilitating homogeneous chemical reactions. Furthermore, 1,4-BDT interacted with Li metal, resulting in the creation of a smooth and enduring SEI layer through subsequent reactions. The resultant organic Li salt Li2-1,4-BDT in the electrolyte was deposited alongside other Li compounds on the Li metal anode, contributing to the SEI layer formation (Fig. 7c). This layer facilitates ion transfer while impeding electron flow, safeguarding the Li metal anode and ensuring stable cycling. Consequently, LSBs with covalently stabilized sulfur maintained a substantial capacity retention of 67.5% over 500 cycles, exhibiting a reversible capacity of 909.3 mAh g−1. Also, the Li–S pouch cell shows an initial specific energy of 349 Wh kg−1 and remains 298 Wh kg−1 after 26 cycles at a discharge current of 300 mA (Fig. 7d).
a Schematic illustration of anode protection and capacity enhancements by adding SOCl2. b Long term cycling curves using electrolyte with or without SOCl2 additives at 1600 mA g.−1 (Reprinted with permission from [128], 2019, Elsevier). c Reaction mechanism in the initial discharge and recharge process with 1,4-BDT. d Cycling performance of the Li-S pouch cell with 1,4-BDT at a current of 300 mA (Reprinted with permission from [129], 2021, American Chemical Society). e Schematics of the formation of SEI with and without BTB additive f schematics of SEI preformation in the LiPS electrolyte (left) and the optical images of LiPS electrolyte with a Li foil after 0 and 16 h in the visualized test (right) blue: organosulfur containing SEI, red: routine SEI. g cycling performance of LSB at 0.1 C with and without BTB (Reprinted with permission from [131], 2020, Wiley–VCH). h Molecular orbital energies of the different solutes and solvents. i CE of Li metal anodes in LiTFSI/KPF6 and schematic illustration of Li plating process in LiTFSI/KPF6 (inner) (Reprinted with permission from [132], 2020, American Chemical Society)
In addition to manipulating the SEI layer components, safeguarding the Li metal anode against LiPSs or ensuring uniform Li distribution through electrostatic shielding is of paramount importance [130]. Wei et al. explored the use of 3,5-bis(trifluoromethyl)thiophenol (BTB) as an electrolyte additive to establish an organosulfur-containing SEI for LiPS shielding [131]. This specific SEI-containing organosulfur shields the Li metal anodes from detrimental side reactions with LiPSs, significantly enhancing the uniformity of Li plating and diminishing the overpotential during both the plating and strip** processes (Fig. 7e). The effectiveness of LiPS shielding was verified by performing SEI and visualizing the test (Fig. 7f). In practical application under specified conditions (4.5 mgs cm−2, 5.0 μL mgs−1, 50 μm Li), LSBs exhibited improved cycling stability, completing 82 cycles with an electrolyte containing 80 mM BTB in 1 M LiTFSI in DOL/DME (1:1, by volume) with 2% LiNO3, in contrast to 42 cycles with a blank electrolyte (Fig. 7g). Li et al. highlighted the enhancement in the cycling stability of LSBs upon the introduction of 0.01 M potassium hexafluorophosphate (KPF6) into a 2 M LiTFSI/ether-based electrolyte [132]. This modified electrolyte demonstrated sustained cycling performance beyond 200 cycles, achieving high Li utilization of up to 33.3%. This enhancement resulted from the combined effect of the self-healing electrostatic shield effect attributed to K+ cations and the formation of a LiF-rich SEI derived from PF6− anions. Notably, Density Functional Theory (DFT) calculations revealed the lower LUMO energy of KPF6 compared to other electrolyte components, leading to the preferential decomposition of PF6− anions, thereby promoting the formation of an underlying SEI enriched in LiF (Fig. 7h). Simultaneously, the electrostatic shield provided by the K+ ions facilitates regulated Li+ distribution, ensuring uniform Li electrodeposition. Consequently, the cell employing the modified LiTFSI/KPF6 electrolyte exhibited a high CE, exceeding 98.8% after 20 cycles, maintained stability over 200 cycles (Fig. 7i).
5.2 Sparingly solvating electrolytes
The inevitable diffusion of LiPSs driven by concentration gradients leads to the corrosion of the Li metal anode. Reducing the solubility of LiPSs in the electrolyte can effectively reduce the high reactivity between the Li metal anode and LiPSs, ultimately enhancing the CE. The solubility of LiPSs in an electrolyte is intricately linked to the solvent properties, particularly its polarity and Lewis basicity [133]. Solvents with lower dielectric constants or Gutmann donor numbers exhibit reduced polarity and weaker Lewis basicity, resulting in diminished coordination with Li+ in LiPSs [134]. Consequently, when the interaction force between the solvent and the LiPSs was weaker than the binding force within the LiPSs, the solubility of the LiPSs in the electrolyte decreased. Employing a solvent with a lower solubility for LiPSs or adjusting the solvation structure can effectively limit the dissolution of LiPSs, leading to enhanced stability of the Li metal anode.
5.2.1 Solvent engineering
To decrease the solubility of LiPSs in solvents, various strategies such as increasing the carbon-to-oxygen ratio (C/O ratio) [135,136,137,138,139], introducing steric hindrance, or incorporating electron-withdrawing groups into the solvent can be employed [140, 141]. Sun et al. demonstrated the effectiveness of a high C/O ratio for reducing the dielectric constant of a solvent, thereby yielding a less polar solvent [137]. They investigated ethers with high C/O ratios, namely methyl tert-butyl ether (MTBE, C/O = 5, εr = 4.38) and diisopropyl ether (DIPE, C/O = 6, εr = 3.88), used as co-solvents in a DOL/DME (1:1, by volume). The solubility of Li2S8 in DOL/DME was approximately 0.5 M, whereas in MTBE and DIPE, when they constituted 50% of the electrolyte, it reduced drastically to 20 mM and 4 mM, respectively (Fig. 8a). Furthermore, the linear isomer of MTBE, methyl butyl ether (MBE), can dissolve up to 39 mM of Li2S8, suggesting that the steric hindrance introduced by the alkyl groups hinders Li+ solvation. Employing an electrolyte composed of DME/DOL/DIPE (25:25:50, by volume) resulted in a 15% enhancement in the CE of the LSB compared of the reference. Analysis of the anode after cycling indicated diminished damage to the Li metal anode and the presence of reduced sulfur in the SEI layer, signifying the inhibition of the shuttle effect. Weller et al. utilized hexyl methyl ether (HME) as a co-solvent with DOL in LSBs [139]. UV–vis measurements exhibited that only 50 mM of Li2S8 dissolved in a 2 M LiTFSI HME/DOL (9:1, by volume) (Fig. 8b). This HME-containing electrolyte effectively restrained the dissolution of LiPSs and successfully suppressed detrimental LiPS shuttling.
a Molecular structure of MTBE and DIPE (upper) and room temperature Li2S8 solubility vs the volume percentage of high C/O ratio ether cosolvents mixed with DOL/DME (1:1, by volume) (lower) (Reprinted with permission from [137], 2018, American Chemical Society). b Addition of 5 mM, 7.5 mM, 10 mM, 20 mM Li2S8 and saturated Li2S8 in 2 M LiTFSI in HME/DOL (9:1, by volume) after stirring overnight (Reprinted with permission from [139], 2019, Wiley–VCH). c LiPS solubility test in fluorinated electrolyte solvents: 1.0 M Li2S8 dissolved in electrolytes (Reprinted with permission from [143], 2015, American Chemical Society)
The incorporation of electron-withdrawing groups into solvents diminishes their Lewis basicity. Hydrofluoroethers (HFEs), which possess strong electronegativity and an electron-withdrawing effect, exhibit decreased affinity toward Li+, effectively suppressing the dissolution of LiPSs. The extent of fluorination and arrangement of the fluoroalkyl groups in the HFEs significantly influenced their solvating capacity for LiPSs. The inclusion of highly fluorinated HFEs exhibits enhanced capacity retention in contrast to less fluorinated HFE cosolvents [142]. This phenomenon arises from the increased capacity of the fluoroalkyl group to attract electrons, correlating with the higher degree of fluorination within the HFE molecules. Azimi et al. observed that increasing the content of 1,1,2,2-tetrafluorethyl-2,2,3,3-tetrafluoropropyl ether (TTE) led to a notable reduction in the solubility of LiPSs [143]. The solubility of Li2S8 in 1 M LiTFSI in TTE/DOL (1:1, by volume) was approximately 2 mM (Fig. 8c). Furthermore, the interaction between TTE and Li metal generates a LiF-rich SEI layer, effectively curbing the undesired reactions of dissolved LiPSs with Li metal. The electrolyte formulation of TTE/DOL (1:1, by volume) displayed a notably higher CE of 97% that of the conventional DOL/DME (1:1, by volume) electrolyte in LSBs. Similarly, Talian et al. investigated an electrolyte composition containing 1,2-(1,1,2,2-tetrafluoroethoxy)ethane (TFEE), a fluorinated ether, in combination with DOL to reduce LiPS solubility [144]. For 1 M LiTFSI in the TFEE/DOL electrolyte, the solubility of Li2S8 was less than 2 mM. In addition, the Li–S pouch cell with 1 M LiTFSI in TFEE/DOL (1:1, by volume) achieved capacities exceeding 1200 mAh gs−1 and a CE just below 97%, indeed without the use of any LiNO3. Su et al. additionally highlighted that the positioning of fluorine atoms has a more substantial impact on solvation than the number of fluorine atoms [145]. The presence of both α- and β- substituted fluoroalkyl groups in HFEs resulted in their exhibiting the least Li solvating capacity and the highest tendency to mitigate the dissolution of LiPSs.
5.2.2 Adjusting the concentration of electrolytes and solvation structure
Unbound solvent molecules that are not involved in the solvation of Li+, contribute to the dissolution of LiPSs. Increasing the concentration of Li salts reduces the quantity of free solvent, thereby decreasing the solubility of the LiPSs. In high-concentration electrolytes (HCEs), often described as solvent-in-salt systems, surplus salt anions form contact ion pairs (CIPs) or aggregates (AGGs) that bind to more than one Li+, resulting in a notable reduction in the amount of unbound solvents [146]. Moreover, CIPs and AGGs can shift the LUMO from the solvent to the salt. Consequently, an SEI layer was generated by the reduction products of the salt, primarily composed of inorganic constituents, which effectively hindered the formation of Li dendrites. This approach curtails the shuttle effect and Li dendrite formation owing to the decreased solubility of LiPSs and the inorganic-rich SEI layer, which consequently enhances the performance of LSBs.
Suo et al. introduced a highly concentrated “solvent-in-salt” electrolyte category designed for LSBs [147]. Employing 7 M LiTFSI in DOL/DME (1:1, by volume) at near-saturation levels, they achieved minimal dissolution of sulfur and lithium sulfide (Li2S) over 18-day duration. As an increase in LiTFSI concentration from 2 to 7 M, the CE of LSBs significantly rose from 85% to nearly 100%. After 280 cycles, the 7 M LiTFSI-containing electrolyte displayed the least amount of damage to the Li metal compared with the electrolytes with lower concentrations. Additionally, the SEM images indicate that the solvent in the salt electrolyte system can effectively reduce corrosion and suppress the formation of Li dendrites. Zheng et al. used stable 12 M lithium bis(fluorosulfonyl)amide (LiFSI) in a DME electrolyte formulation for LSBs, ensuring enhanced safety and stability (Fig. 9a) [53]. This high concentration effectively curtailed Li dendrite growth on the anode and suppressed LiPS shuttle reactions on the cathode side, yielding impressive CE (99.7% for sulfur cathode and 99.2% for Li anode). Pang et al., on the other hand, observed that reducing the ratio of solvent to salt alters the sulfur reaction pathway, transitioning it from a dissolution–precipitation system to a quasi-solid-state conversion regime [138]. The diethylene glycol dimethyl ether system (G2)/LiTFSI notably differs from the triethylene glycol dimethyl ether (G3)/LiTFSI and tetraethylene glycol dimethyl ether (G4)/LiTFSI systems due to the chain length, enabling a complete wrap** around the TFSI- anion and forming an extended network structure. At room temperature, the solubility of Li2S6 in G2/LiTFSI is only 2 mM, which effectively mitigates the shuttle effect and supports uniform Li deposition. As a result, a Li–S pouch cell with a low E/S ratio of 5 µL mg−1 in the G2/LiTFSI (0.8:1, by molar) showed a stable capacity of 720 mAh g−1 over 100 cycles, whereas the cell in the G2/LiTFSI (7:1, by molar) showed a rapid capacity degradation after only 20 cycles. However, deploying such highly concentrated electrolytes leads to reduced Li+ conductivity because of their elevated viscosity, which affects the energy density of LSBs. Additionally, the increased salt volume significantly increases the electrolyte cost.
a Schematic illustration of 12 M LiFSI/DME for LSB (Reprinted with permission from [53], 2018, Elsevier). b Effect mechanism of MDHCE for LSBs (Reprinted with permission from [173]. CMK-3 is distinguished by its structure of hollow carbon nanorods and 3 nm channel voids, which effectively accommodate sulfur through melt-diffusion at 155 ℃. This method ensures a uniform sulfur distribution and close contact with the carbon structure.
However, carbon-only sulfur hosts have limitations owing to their reliance on physical interactions with LiPSs, such as Van der Waals forces and confinement effects, which are not sufficient for effectively capturing dissolved LiPSs. Consequently, research has evolved toward incorporating other components with carbon that induce chemical adsorption effects on LiPSs or catalytic effects to accelerate the conversion reactions of LiPSs, thereby suppressing the LiPS shuttle effect [174,175,176,177]. Previous studies predominantly focused on using polar metal compounds to achieve chemical adsorption and catalytic effects [178]. Recent studies have also explored effective catalysts, including heterostructures and single-atom catalysts [184, 185].
Wang et al. developed a unique structure using an oxygen-deficient niobium oxide (Nb2O5-x) framework with a 3D ordered microporous (3DOM) architecture and embedded carbon nanotubes (CNTs) [186]. This highly porous, open architecture ensures sufficient exposure of the active interfaces. Concurrently, defect engineering not only increases the electrical conductivity of Nb2O5 but also enhances its chemical interactions with LiPSs by reducing the bond length between Li in LiPSs and O in Nb2O5-x, thereby improving the reaction kinetics and LiPS adsorption capability. Additionally, the embedded CNTs form a highly conductive network, significantly enhancing the electrical conductivity of the sulfur host (2.46 × 10–3 S cm−1). This innovative design effectively suppressed the shuttle behavior of LiPSs and accelerated their conversion kinetics (Fig. 11a). As a result, LSBs based on the S-Nb2O5-x/CNTs cathode demonstrated exceptional cyclability, retaining a high capacity of 847 mAh g−1 after 500 cycles, and a notable rate capability of 741 mAh g−1 at 5 C.
a Schematic illustration of Nb2O5-x/CNTs (Reprinted with permission from [186], 2020, Wiley–VCH). b Geometry configuration of Li2S6 binding to HEMO-1 (the oxygen, nickel, magnesium, zinc, copper, cobalt, Li and sulfur atoms are marked with red, white, orange, green, blue, purple, luminous yellow and light green, respectively.) (Reprinted with permission from [187], 2019, Elsevier). c Charge/discharge voltage profiles of CNF@V5S8/S, CNF@VS2/S and CNF/S cathode at 0.2 C (Reprinted with permission from [193], 2021, Elsevier). d Illustration of the mechanisms during redox reaction for NC-S and NC/MoS3-S NBs-based batteries (Reprinted with permission from [216], 2023, American Chemcial Society)
**–Coupling–Conversion” enables high-efficiency Nb single-atom catalysis for Li–S batteries. J Am Chem Soc. 2023. https://doi.org/10.1021/jacs.2c10345 ." href="/article/10.1007/s43938-024-00045-w#ref-CR216" id="ref-link-section-d9170602e3972">216]. The cornerstone of their strategy was the “trap**-coupling-conversion” mechanism, which effectively anchors and converts LiPSs, thus protecting Li metal anode and enhancing battery performance. The distorted Nb-N sites, featuring unfilled antibonding d orbitals, were coupled with the p orbitals of sulfur, facilitating strong interactions between the catalyst and LiPSs. Employing the Nb-SAs@NC catalyst suppressed surface deterioration of the Li metal anode. Atomic force microscopy (AFM) analysis revealed that the cells with the Nb-SAs@NC cathode had smoother and more compact Li metal anode surfaces than those with the S@NC cathode, indicating effective LiPS trap** (Fig. 13h). As a result, the S@Nb-SAs@NC cathode achieved a reversible capacity of 807.4 mAh g−1 over 600 cycles, corresponding to a capacity retention of 87.1%.
7 Separator/interlayer engineering
7.1 Separator engineering
In LSBs, the separator is positioned between the cathode and anode and directly interacts with the active material of the cathode and Li at the anode. Therefore, the mitigation of the LiPS shuttle effects and Li metal degradation can be achieved simultaneously by modifying the separator, making this method efficient. Consequently, extensive research has been conducted on separator engineering to enhance the cycling stability through interactions with LiPSs and Li [217, 218]. Such studies can be categorized into (1) LiPS adsorption, (2) Li plating regulation, and (3) direct interactions with Li. However, modifying separators involves adding additional materials on the separator, which increase its thickness and mass, leading to a reduction in energy density, an increase in internal resistance, and the use of an electrolyte [219]. Therefore, recent studies have actively pursued thin and lightweight separators that retain these functionalities [220, 221]. The details of these studies are presented in the following sections.
7.1.1 LiPS adsorption
In typical LSBs, polypropylene (PP) separators with pore sizes of approximately 70 nm are commonly used [222]. However, the average size of the LiPSs is several nanometers, which allows them to permeate the separator and interact with Li, leading to undesirable reactions [223]. To mitigate such adverse interactions between the LiPSs and Li, researchers have focused on introducing additional materials with adsorptive and catalytic capabilities into the separator to inhibit the LiPS shuttle effect [224]. Moreover, the LiPSs adsorbed on the separator can be reactivated and contribute to capacity retention [225]. In parallel with cathode engineering, extensive research has been conducted on metal compounds, heterostructures, and single-atom catalysts [226, 227]. The introduction of these materials possessing both adsorption and catalytic capabilities significantly inhibit the LiPS shuttle effect, thereby preventing the diffusion of LiPSs to the Li metal anode and effectively protecting against Li corrosion caused by LiPS.
Zou et al. developed In2O3-x nanoparticles combined with carbon spheres (CS), resulting in defect-rich electrocatalysts that enhance the chemical adsorption and catalytic conversion of LiPSs [228]. DFT calculations confirmed that In2O3-x exhibits a higher adsorption energy for LiPSs than In2O3 which demonstrates the efficacy of defect engineering. In cells equipped with the In2O3-x@CS-0.6/rGO separator, a low-voltage hysteresis of approximately 150 mV was observed after cycling for 500 h at a current density of 5 mA cm−2 and an areal capacity of 5 mA cm−2 (Fig. 14a). Liu et al. developed a novel strategy to suppress the LiPS shuttle effect in LSBs by utilizing phosphorus-doped metal–organic framework-derived CoS2 (P-CoS2) nanoboxes [228], 2022, Elsevier). b Proposed adsorption schemes of Li2S6 on CoS2 and P-CoS2 surfaces. c Voltage curves under the self-discharge test of the cells with P-CoS2/CNTs@Celgard and CoS2/CNTs@Celgard at 0.1 C (Reprinted with permission from [231]. Created through a straightforward one-pot synthesis process, this electrocatalyst consists of Co3ZnC-embedded carbon submicrospheres anchored on 3D macromesoporous N-doped carbon. The inclusion of Zn cations effectively modulated the active Co2+/Co0 pair, thereby inhibiting the shuttle effect and accelerating the catalytic conversion kinetics of LiPSs. When used as an interlayer to modify commercial separators, Co3ZnC@NC significantly enhanced the cycling stability and discharge capacity of LSBs demonstrating a substantial specific capacity of 805.5 mAh g−1 after 50 cycles at 0.2 C under a high sulfur loading of 4 mg cm−2. Lv et al. introduced a unique VSe2/V2C heterostructure, created from a facile thermal selenization process from V2C [232]. This structure combines the strong affinity of V2C for LiPSs with the electrocatalytic activity of VSe2, and the local built-in electric field at the heterointerface effectively enhances electron/ion transport and boosts the conversion kinetics of LiPSs (Fig. 14e). LSBs equipped with a VSe2/V2C-CNTs-PP separator exhibited remarkable performance, achieving an initial specific capacity of 1439.1 mAh g−1 and maintaining a capacity of 571.6 mAh g−1 after 600 cycles.
**g et al. introduced a defect-rich single-atom catalytic material (Fe-N4/DCS) to enhance the LSBs by addressing the LiPS shuttle effect [233]. Abundant local defects in substrates not only facilitate the dispersion of Fe atoms but also provide stable sites for single atoms. Additionally, DFT calculations demonstrated that Fe atoms in the defect environment strongly adsorbed LiPSs and possessed a lower activation energy for the redox reactions of LiPSs. By incorporating Fe-N4/DCS into a PP separator (Fe-N4/DCS/PP), this study demonstrated that this approach can significantly inhibit LiPS shuttling and accelerate redox reactions. Figure 14f visually demonstrates the superiority of the Fe-N4/DCS separator in preventing LiPS accumulation and corrosion on the Li metal anode compared with conventional PP separators. The LSBs with the modified separator exhibited a remarkable performance improvement, with a capacity decay rate of only 0.03% per cycle at 2 C after 800 cycles. Zhang et al. have developed a novel catalyst (VN1-x@V-NC) featuring single vanadium (V) atoms with V-N4 coordination and vanadium nitride nanodots characterized by substantial nitrogen vacancies (VN1-x) [234]. Both theoretical calculations and experimental results have demonstrated that the V-N4 and VN1-x sites have catalytic effects of Li2S formation and decomposition, respectively. Moreover, VN1-x exhibits a strong affinity for soluble polysulfides through V-S and Li-N bonding interactions, with LiPSs being preferentially adsorbed near the nitrogen vacancies. This configuration effectively mitigates the LiPS shuttle effect. Consequently, when Li–S pouch cells are equipped with the VN1-x@V-NC modified separator, they demonstrate an impressive initial discharge capacity of 1086.5 mAh g−1 and maintain stable cycling performance, retaining 86.35% of their initial capacity after 50 cycles at 0.1 C (Fig. 14g).
7.1.2 Li plating regulation
In LSBs, the separator serves as a pathway for Li+ to move between the anode and the cathode. If the mobility within the separator is low, a cation concentration gradient is formed at the anode, facilitating the formation of dendrites [235]. Conversely, a high Li+ mobility can create a homogeneous Li+ flux and induce uniform Li plating, thereby suppressing dendrite formation [236]. Thus, enhancing the Li+ transfer within the separator is an efficient strategy for regulating Li plating and inhibiting dendrite growth. In addition, recent studies have reported that the presence of functional groups such as polar and negatively charged groups can further mitigate the LiPS shuttle effect. Bifunctional separators exhibiting these two effects are discussed below.
Wang et al. introduced a novel anionic metal–organic framework (MOF)-based bifunctional separator, named MMMS, which addresses the dendrite issue in Li metal anodes for LSBs [237]. The MMMS, fabricated using UiO-66-SO3Li and poly(vinylidene fluoride) (PVDF) through a mixed-matrix membrane approach, featured well-defined anionic Li+ transport tunnels composed of abundant sulfonate (-SO3−) groups (Fig. 15a). These tunnels facilitate homogeneous Li deposition, which is crucial for stabilizing Li-metal anodes. Meanwhile, the sulfonate groups exhibit electrostatic repulsion forces against the LiPS anions, thereby playing a role in suppressing the shuttle effect of LiPS. Li et al. enhanced the performance of LSBs by engineering a stable electrode-separator interface [238]. This was achieved by coating both surfaces of a commercial Celgard separator with an ultrathin conductive polymer nanolayer of polypyrrole (PPy) using a simple and scalable in situ vapor-phase polymerization process (Fig. 15b). This modification results in only a minor increase in the overall mass and volume of the separator (~ 0.13 mg cm−2 of mass loading). The hydrophilic nature of PPy enhances the electrolyte uptake, which facilitates uniform Li+ flux across the separator, leading to uniform Li metal strip** and plating. Furthermore, the conducting properties of PPy facilitated electron transfer, enhancing the redox reaction of LiPSs on the cathode side. Consequently, the modified separator demonstrated superior performance, as evidenced by a low and stable overpotential of less than 30 mV for over 250 h at a current density of 1 mA cm−2 with an areal capacity of 3 mAh cm−2 during the Li strip** and plating tests (Fig. 15c).
a Schematic illustration of the MMMS for regulating Li deposition and blocking LiPS shuttle in LSBs (Reprinted with permission from [237], 2020, Wiley–VCH). b Schematic illustration of coating a PPy ultrathin nanolayer on both surfaces of the separator using a facile vapor-phase polymerization process. c Voltage profile of Li||Li symmetric cells with regular Celgard separator and PPy modified separator at the current density of 1 mA cm-2 with the areal capacity of 3 mAh cm-2 (Reprinted with permission from [238], 2019, Elsevier). d Schematic illustration of cycled Li behaviors with PP and COF-CN-S@PP separator. e In situ optical microscopy observations of Li/PP/Li and Li/COF-CN-S@PP/Li batteries during the Li plating/strip** process at 1 mA cm-2 with 0.5 mAh cm-2 (Reprinted with permission from [239], 2023, Wiley-VCH). f Calculated adsorption energies (Eads) of PP and pVIDZ with soluble LiPS species (Li2S4, Li2S6, and Li2S8) (Reprinted with permission from [240], 2021, American Chemical Society). g Schematic representation and cycling performance of the flexible Li-S pouch cell (Reprinted with permission from [243], 2022, John Wiley and Sons)
An et al. designed a bifunctional separator (COF-CN-S) using an asymmetric covalent organic framework (COF) with strong cyanide groups (-CN) and polysulfide chains (-S) for LSBs. [239]. Polar functional groups (nitrile) and electronegative sulfur groups suppress LiPS shuttle effects through adsorption and electrostatic repulsion, respectively. Meanwhile, the lone pairs of cyano groups are inclined to coordinate with Li+, which enhances the Li+ transport capability via reversible coordination bonds. These characteristics regulated the dynamic behavior of LiPSs and Li+, thus inhibiting the shuttle effects of LiPSs and dendrite growth (Fig. 15d). In situ optical microscopy images provided a clear contrast between the two types of separators, COF-CN-S@PP and PP. The COF-CN-S@PP separator exhibited superior control over the Li deposition, leading to more uniform and dendrite-free Li layers (Fig. 15e). Lim et al. employed initiated chemical vapor deposition (iCVD) to uniformly coat polyvinylimidazole (pVIDZ) onto a separator, creating a ultrathin (70–100 nm) and ultralight-weight (0.055 mg cm−2) bifunctional separator for LSBs [240]. The imidazole functional groups in pVIDZ interacted with the anions of LiTFSI, enhancing Li+ mobility and effectively inhibiting dendrite formation. Additionally, DFT calculations confirmed that pVIDZ offers a high LiPS adsorption energy compared to the PP separator, with significant LiPS shuttle effects (Fig. 15f). Consequently, LSBs employing a separator with pVIDZ delivered a specific capacity of 881 mAh g−1 at 0.1 C.
Fan et al. presented a synergistic functional separator composed of graphene quantum dots (GQDs) and polyacrylonitrile (PAN) on a polypropylene (PP) base (GQDs-PAN@PP separator) [241]. The quantum confinement effect of GODs and polar functional groups acts as a lithiophilic mediator that induces uniform Li+ nucleation and deposition. Additionally, the 3D network formed by the electrospun nanofibers combined with the polar functional groups of the GQDs effectively inhibited the shuttling of LiPSs and enhanced sulfur utilization. As a result, Li||Li symmetric cell with the GQDs-PAN@PP separator demonstrates stable cycling for over 600 h, and LSBs with this separator achieve high stability and desirable sulfur electrochemistry, including a high reversibility of 558 mAh g−1 for 200 cycles and a low fading rate of 0.075% per cycle after 500 cycles at 0.5 C. Yan et al. implemented a versatile asymmetric separator composed of lithiated-sulfonated porous organic polymer (SPOP-Li) and Li6.75La3Zr1.75Nb0.25O12 (LLZNO) layers to LSBs [243]. This method facilitates the electronic interactions at the heterointerfaces between Pt SAs/In2S3 and Ti3C2, which induce charge distribution at these interfaces. The Pt SAs/In2S3/Ti3C2@PP separator enhances the homogeneous distribution of Li+ flux, attributed to the hetero-interfacial electronic enhancement effects between Pt SAs/In2S3 and Ti3C2, thereby inhibiting Li dendrite growth even at a high current density of 5 mA cm−2. Consequently, Li–S pouch cells equipped with the Pt SAs/In2S3/Ti3C2@PP separator have achieved an areal capacity of 5.54 mAh cm−2 at a discharge rate of 0.2 C (Fig. 15g).
7.1.3 Direct interaction with anode
As discussed previously, the separator is in contact with the Li metal anode and can interact directly with Li. Therefore, functionalization of the separator can induce beneficial interactions between the separators and Li. Various mechanisms of interaction with the anode, such as regulating Li growth, reconstructing the SEI layer, and rejuvenating inactive or dead Li, have been suggested to protect the anodes of LSBs [244]. Research related to these mechanisms and their implementation in protecting the anode is discussed in the following section. These methods significantly increase the cycling stability of LSBs.
Song et al. introduced an innovative strategy using Mo-containing polyoxometalate (POMs)-modified separators to inhibit Li dendrite growth in LSBs [245]. Specifically, the optimized Dawson-type POM, (NH4)6[P2Mo18O62]·11H2O (P2Mo18), is employed for its strong oxidizability. When Li dendrites form and come into contact with the separator, P2Mo18 acts as a “dendrite-killer,” oxidizing Li0 into Li+, thereby mitigating the risks posed by dendrites. This prevents the formation of dead Li, which degrades the CE and cycling performance. Moreover, the reduced state of P2Mo18 can be re-oxidized, allowing its reusability. This mechanism effectively prevents short circuits and potential spontaneous combustion owing to uncontrolled dendrite growth. The Li||Li symmetrical cell with the P2Mo18 modified separator demonstrates exceptional cyclic stability for over 1000 h at 3 mA cm−2 and 5 mAh cm−2 (Fig. 16a). Furthermore, the assembled LSBs maintain a superior reversible capacity of 600 mAh g−1 after 200 cycles at 2 C.
a Galvanostatic cycling of Li||Li symmetric cells with POMs modified separators at 3 mA cm−2 with areal capacity of 5 mAh cm−2 (inset: enlarged diagrams at different periods of the cycling) (Reprinted with permission from [245], 2023, Wiley-VCH). b Scheme portraying the systematic evolution of SEI in the GQDs-modified Li symmetrical cell from the fresh cell to continuous plating-strip** cycles, where GQDs and Li and its salts undergo synergistic interaction to realize smooth and near F-rich SEI (Reprinted with permission from [246], 2022, Wiley-VCH). c Schematic illustration of Li deposition processes of PP@H-PBA, PP@S-PBA, and PP (Reprinted with permission from [247], 2023, Wiley-VCH)
Senthil et al. reported in situ restructured artificial SEI layer characterized by ultrasmooth, thin, and F-rich properties using a site-specific hydroxyl-functionalized graphene quantum dot (GQDs)-coated PP separator [246]. This artificial SEI layer was formed by the synergistic interaction of the plating-borne Li and its species, guided by the hydroxyl-functionalized GQDs (Fig. 16b). Li strip** triggers the dissolution of Li and the decomposition of the electrolyte, whereas Li plating facilitates its redeposition on the exposed Li surfaces. At this juncture, the synergistic interaction between the electron-rich GODs and electron-deficient Li promotes uniform redeposition across the Li surface derived from controlled growth, realizing a smooth and near F-rich SEI layer that affords uniform Li+ flux over the Li metal anode surface.
Liu et al. improved LSB performance by utilizing an Fe-Co-based Prussian blue analog (H-PBA) with a hollow and open framework to modify a commercial PP separator (PP@H-PBA) [247]. The macroporous structure of H-PBA and the open framework guided the growth of Li dendrites through space confinement. Moreover, the positive Fe/Co sites in the H-PBA, which are influenced by polar cyanide (− CN) groups, help in reactivating inactive and dead Li (Fig. 16c). As a result, Li|PP@H-PBA|Li symmetric cells exhibit long-term stability at 1 mA cm−2 for 1 mAh cm−2 over 500 h and LSBs with PP@H-PBA delivered stable cycling performance at 500 mA g−1 for 200 cycles.
7.2 Interlayer engineering
Separator engineering typically involves designing new types of functional separators or modifying existing separators through physicochemical hybridization or functionalization with other materials such as carbons or inorganic materials, ensuring intimate contact between the functional layer and the separator. In contrast, an interlayer refers to a freestanding film inserted between the separator and the electrode [248]. Interlayers are generally divided into two categories: cathodic interlayers, which are placed between the cathode and separator, and anodic interlayers, positioned between the anode and separator. The methods used to protect the Li metal anode of these two types of interlayers differ significantly. Detailed explanations and relevant research are discussed in the following section.
7.2.1 Cathodic interlayer
The primary function of the cathodic interlayer, positioned between the cathode and the separator, is to mitigate the LiPS shuttle effect. Carbon-based materials have been considered promising candidates for the cathodic interlayer owing to their lightweight, high electrical conductivity, ease of fabrication, and stability [249,250,259,260,261].
** processes. These benefits are evidenced by scanning SEM images of Li metal anodes after 10 cycles with a current limit of 3 mAh cm−2 (Fig. 18a). Consequently, LSBs equipped with the CP interlayer demonstrate exceptional cycling stability and reversibility, achieving an initial capacity of over 1200 mAh g−1 and maintaining 600 mAh g−1 after 150 cycles (Fig. 18b).
a Top view and cross-sectional SEM images of bare Li foil and Li-CP after 10 cycles with a 3 mAh cm−2 capacity limit. b Cycling performance of full cells using bare Li foil and Li-CP (Reprinted with permission from [263], 2018, Elsevier). c Contact angle images of O-Ti3C2@CNF. d Optical microscopy study of in situ Li plating behavior of O-Ti3C2@CNF and bare Li metal. e Cycling performance under a sulfur loading of 6.7 mg cm-2 and an E/S ratio of 6 µL mg-1 (Reprinted with permission from [264]. 2022, Elsevier)
He et al. developed a porous carbon nanofiber integrated with an oxygen-rich Ti3C2 MXene nanosheet (O-Ti3C2@CNF) based on first-principles calculations [264]. This O-Ti3C2@CNF interlayer effectively reduces the over-potential for Li nucleation and growth by mitigating the tip effect and lithiophilic properties of the O-Ti3C2 MXene, resulting in a dendrite-free Li metal anode. Additionally, the improved electrolyte affinity of the O-Ti3C2@CNF enhances compatibility at the electrode/separator interface, which is particularly crucial under conditions of a low E/S ratio (Fig. 18c). In situ visual monitoring through an optical microscope confirmed the inhibition of Li dendrite formation on the O-Ti3C2@CNF. It was observed that Bare Li began to deposit on the lithium anode at a constant current density of 2 mA cm−2, with surface bulging initially visible, followed by gradual dendrite formation after 30 min. In contrast, the O-Ti3C2@CNF displayed a homogeneous and dendrite-free Li structure across its entire 3D configuration (Fig. 18d). Consequently, when employed as the anodic interlayer in LSBs, the O-Ti3C2@CNF enabled the batteries to maintain a reversible areal capacity of 5.2 mAh cm−2 across 30 cycles, with a high sulfur loading of 6.7 mg cm−2 and a low E/S ratio of 6 µL mg−1 (Fig. 18e).
8 Conclusion and perspectives
In this paper, we review recent studies on the protection of Li metal anodes in LSBs, categorizing them as anode, electrolyte, cathode, and separator/interlayer engineering. In LSBs, Li metal anode faces several challenges: the formation of Li dendrites & dead Li, high reactivity with electrolytes & LiPSs. Numerous studies have been conducted to protect Li metal anodes because these problems are aggravated under the practical conditions of high sulfur load, low E/S ratio and limited Li anodes. We summarized the performance of the practical pouch cells mentioned in our review article according to the component (Table 1).
However, research on anode protection under the practical condition of high sulfur loading (> 6 mg cm−2), low E/S ratio (< 3 µL/mg), and N/P ratio (< 2) remains insufficient. Therefore, to achieve LSBs with high energy density, considerations beyond the current scope must be addressed for each component of the battery. Therefore, we present the limitations of this study and suggest research directions for each component to achieve practical LSBs.
-
Anode
Two predominant approaches have been explored for modifying Li-metal anodes: the integration of artificial SEI layers and the use of host materials for uniform Li deposition. For practical LSBs, Li metal anode must be modified by a scalable process and the thickness of Li metal must be kept below 50 µm. For application in pouch cells, the modification must be applied equally on both sides of the Li metal. Additionally, there are still areas in which basic research needs to be conducted. For example, forming an artificial SEI layer on lithium alters the surface work function of Li [265]. This change fundamentally affects reactivity of Li with the electrolyte and LiPSs; however, research into these energy levels is not well-developed. Therefore, anode engineering must consider the work function changes and other fundamental factors. Currently, most strategies applied to LSBs are those that have been used in Li metal batteries, but since LSBs involve a system where LiPSs are dissolved in the electrolyte, different strategies must be established. For instance, a dual-layer approach that includes an elastic layer capable of enduring volumetric expansion/contraction on the Li anode side and a functional layer on the electrolyte side to block LiPSs could be effective. Although many studies have been conducted on Li deposition to suppress dendrite formation and dead Li, the strip** process has not been studied extensively. Therefore, a fundamental study of the strip** process should be conducted, especially considering the characteristics of LSB starting from the discharge. Additionally, research on self-discharge, which occurs even when the cell is not in operation, should be conducted.
-
Electrolyte
Electrolyte engineering is crucial for improving the performance and stability of LSBs. To protect the Li metal anode, many sparingly solvating electrolytes with low LiPS solubility have been studied; however, there are challenges associated with high viscosity and increased overpotential. Conversely, an electrolyte with high LiPS solubility can address these issues but may lead to the degradation of the Li metal anode owing to the severe shuttle effect. Furthermore, the electrolyte may be gelled by a super-high concentration of solubilized polysulfides under lean electrolyte conditions. To achieve practical LSBs, a next-generation of electrolytes must be developed to overcome the aforementioned trade-off while maintaining a low E/S ratio. Additionally, researchers are investigating various additives to form a desirable SEI layer. As discussed in Sect. 3.2, LiPSs exhibit an oxidative nature, which leads to their more rapid reduction compared to other electrolyte components, resulting in the formation of a Li2S2/Li2S-rich SEI layer. Therefore, it is necessary to develop effective additives that not only possess lower LUMO energy level to ensure more rapid reduction than LiPSs but also form desirable SEI layer which has high mechanical properties and Li+ conductivity. Another approach of additives involves direct chemical interaction with LiPSs to elevate their LUMO energy level that can modify the reactivity towards the Li metal. These additives should significantly raise the LUMO energy level of LiPSs to reduce their oxidative nature while maintaining original LiPS conversion reaction. In this way, the components of the electrolyte actively interact with LiPSs, significantly influencing their characteristics. Therefore, rather than solely focusing on the Li metal conducted by previous studies, a comprehensive consideration of the impacts on both LiPSs and the Li metal anode is essential for electrolyte design.
-
Cathode
As previously discussed, most cathode studies have been conducted under conditions of high E/S ratios. Consequently, porous materials have been predominantly used as sulfur hosts to increase the contact area with the insulating sulfur and ensure electrical conductivity. However, the use of porous materials with low E/S ratios can lead to the incomplete wetting of the electrode, resulting in Li+ transfer issues. Additionally, a high proportion (~ 30%) of conductive materials in the cathode still reduces the energy density of LSBs. Therefore, it is essential to develop effective sulfur hosts that can ensure electrical conductivity with minimal sulfur content and suitable surface area. Although numerous experimental and computational studies have demonstrated the effects of catalysts on the adsorption and conversion of LiPSs, the fundamental and specific mechanisms of adsorption and conversion remain unclear. Hence, it is important to gain an understanding of the adsorption and conversion reactions of LiPSs using methods such as in situ analyses. Based on this understanding, the design of elaborate catalysts can effectively address the LiPS shuttle phenomenon and Li metal corrosion to a minimal extent. Additionally, for practical application in pouch cells, it is necessary to employ facile synthesis methods and enable large-scale production.
-
Separator/interlayer
In LSBs, separators and interlayers interact with both the cathode and the anode, thereby offering a strategy to simultaneously address issues related to the LiPS shuttle effect and Li metal. However, modification of separators and introduction of interlayers can increase the energy density of LSBs. To achieve LSBs with high energy densities, it is necessary to develop thin and lightweight separators and interlayers that retain these beneficial effects. However, thin separators exhibit reduced capability to suppress the LiPS shuttle effect due to decreased surface area and thickness, and they are vulnerable to internal short circuits caused by Li dendrite formation. Similarly, thin interlayer is likely to fail in suppressing LiPS shuttle effect and Li dendrite formation. Therefore, to overcome this trade-off, there is a need to develop an effective separator and interlayer that exhibits excellent LiPS shuttle effect suppression capability with robust mechanical strength while maintaining thin and lightweight properties. Furthermore, the surface area, pore volume, and surface functional groups of separators and interlayers significantly impact electrolyte wettability, Li+ migration capability, and the required amount of electrolyte. Therefore, these factors must be considered in the design of separators and interlayers. Such effects are more pronounced in practical pouch cells operating at low E/S ratios (< 3 µL/mg) with limited electrolyte amount and in high viscosity environments, thus requiring thorough consideration to effectively protect Li metal anode. Finally, for practical application in pouch cells, it is essential to ensure large-scale production and uniform properties across large areas.
Compared to traditional LIBs, LSBs offer a significantly higher energy density and cost efficiency. However, the realization of these advantages requires practical conditions. Under such conditions, the operational environment of LSBs changes drastically, and issues such as Li metal challenges become more pronounced. Furthermore, to satisfy the practical conditions, the required properties of each battery component are changed. However, many studies on LSB have only been conducted at the coin cell stage, neglecting various problems that may occur in the Li metal anode. The Li metal anode not only corrodes when reacting with the electrolyte, but also experiences extreme capacity decay when reacting with high concentration LiPSs in the pouch cell of practical condition. Unfortunately, this issue of Li metal anode corrosion is not treated as importantly in LSB studies as it is in lithium metal battery studies. Existing research lacks direct investigation into the corrosion of Li metal by LiPSs, with most studies focusing on characterizing the morphology of Li via SEM or assessing the degradation of Li metal through XPS. Consequently, a fundamental understanding of lithium metal surface corrosion by LiPS is required, paving the way for the development of strategies to protect Li metal. Furthermore, Research on dead sulfur formation at the anode is not extensively addressed. Dead sulfur at the anode refers to species that are reduced from LiPSs through the shuttle effect, subsequently deposited, and unable to function as active cathode material, leading to capacity decay [266,267,268]. Although it is important to suppress the reactivity between LiPSs and Li metal anode, as is currently conducted, reactivating the already-formed dead sulfur at the anode using the methods such as additives to re-engage it in electrochemical reactions is also crucial for enhancing the cycling performance of LSBs. Based on these considerations, a comprehensive LSB design that considers the interactions between each component could pave the way for the commercialization of LSBs.
Data availability
Not applicable.
References
Dunn B, Kamath H, Tarascon J-M. Electrical energy storage for the grid: a battery of choices. Science. 2011. https://doi.org/10.1126/science.1212741.
Armand M, Tarascon J-M. Building better batteries. Nature. 2008. https://doi.org/10.1038/451652a.
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci. 2011. https://doi.org/10.1039/C1EE01598B.
Winter M, Barnett B, Xu K. Before Li ion batteries. Chem Rev. 2018. https://doi.org/10.1021/acs.chemrev.8b00422.
Zhou L, Danilov DL, Eichel RA, Notten PH. Host materials anchoring polysulfides in Li–S batteries reviewed. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202001304.
Tian Y, Zeng G, Rutt A, et al. Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and grid decarbonization. Chem Rev. 2020. https://doi.org/10.1021/acs.chemrev.0c00767.
Jan W, Khan AD, Iftikhar FJ, Ali G. Recent advancements and challenges in deploying lithium sulfur batteries as economical energy storage devices. J Energy Storage. 2023. https://doi.org/10.1016/j.est.2023.108559.
Kim J, Lee S, Kim S, et al. Anode-less hybrid Na–CO2 battery with sodium harvesting from seawater for both electricity storage and various chemical production. ACS Energy Lett. 2023. https://doi.org/10.1021/acsenergylett.3c01977.
Park CY, Kim J, Lim WG, Lee J. Toward maximum energy density enabled by anode-free lithium metal batteries: recent progress and perspective. Exploration. 2023. https://doi.org/10.1002/EXP.20210255.
Kim J, Kim J, Jeong J, et al. Designing fluorine-free electrolytes for stable sodium metal anodes and high-power seawater batteries via SEI reconstruction. Energy Environ Sci. 2022. https://doi.org/10.1039/D2EE01295B.
Kim S, Jung H, Lim WG, et al. A versatile strategy for achieving fast-charging batteries via interfacial engineering: pseudocapacitive potassium storage without nanostructuring. Small. 2022. https://doi.org/10.1002/smll.202202798.
Lee J, Kim J, Kim S, Jo C, Lee J. A review on recent approaches for designing the SEI layer on sodium metal anodes. Mater Adv. 2020. https://doi.org/10.1039/D0MA00695E.
He J, Chen Y, Manthiram A. Vertical Co 9 S 8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li–S batteries. Energy Environ Sci. 2018. https://doi.org/10.1039/C8EE00893K.
Wang G, **ong X, **e D, et al. Suppressing dendrite growth by a functional electrolyte additive for robust Li metal anodes. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2019.02.026.
Bi CX, Zhao M, Hou LP, et al. Anode material options toward 500 Wh kg− 1 lithium–sulfur batteries. Adv Sci. 2022. https://doi.org/10.1002/advs.202103910.
Li Z, Jiao S, Yu D, et al. Cationic-polymer-functionalized separator as a high-efficiency polysulfide shuttle barrier for long-life Li–S battery. ACS Appl Energy Mater. 2021. https://doi.org/10.1021/acsaem.1c00281.
Lim WG, Kim S, Jo C, Lee J. A comprehensive review of materials with catalytic effects in Li–S batteries: enhanced redox kinetics. Angew Chem Int Ed. 2019. https://doi.org/10.1002/anie.201902413.
Moon S, Jung YH, Jung WK, Jung DS, Choi JW, Kim DK. Encapsulated monoclinic sulfur for stable cycling of li-s rechargeable batteries. Adv Mater. 2013. https://doi.org/10.1002/adma.201303166.
Fang R, Zhao S, Pei S, et al. Toward more reliable lithium–sulfur batteries: an all-graphene cathode structure. ACS Nano. 2016. https://doi.org/10.1021/acsnano.6b04019.
Xu ZL, Huang JQ, Chong WG, et al. In situ TEM study of volume expansion in porous carbon nanofiber/sulfur cathodes with exceptional high-rate performance. Adv Energy Mater. 2017. https://doi.org/10.1002/aenm.201602078.
Huang Y, Wang W, Shan J, et al. High volumetric energy density Li-S batteries enabled by dense sulfur monolith cathodes with ultra-small-sized sulfur immobilizers. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2020.126076.
Liu F, Lu W, Huang J, et al. Detangling electrolyte chemical dynamics in lithium sulfur batteries by operando monitoring with optical resonance combs. Nat Commun. 2023. https://doi.org/10.1038/s41467-023-43110-8.
Chen Y, Wang T, Tian H, Su D, Zhang Q, Wang G. Advances in lithium–sulfur batteries: from academic research to commercial viability. Adv Mater. 2021. https://doi.org/10.1002/adma.202003666.
Hou LP, Yao N, **e J, et al. Modification of nitrate ion enables stable solid electrolyte interphase in lithium metal batteries. Angew Chem Int Ed. 2022. https://doi.org/10.1002/anie.202201406.
Liu Y, Elias Y, Meng J, et al. Electrolyte solutions design for lithium-sulfur batteries. Joule. 2021. https://doi.org/10.1016/j.joule.2021.06.009.
Li S, Zhang W, Zheng J, Lv M, Song H, Du L. Inhibition of polysulfide shuttles in Li–S batteries: modified separators and solid-state electrolytes. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202000779.
Huang Y, Lin L, Zhang C, et al. Recent advances and strategies toward polysulfides shuttle inhibition for high-performance Li–S batteries. Adv Sci. 2022. https://doi.org/10.1002/advs.202106004.
Zhao W, Lei Y, Zhu Y, et al. Hierarchically structured Ti3C2Tx MXene paper for Li-S batteries with high volumetric capacity. Nano Energy. 2021. https://doi.org/10.1016/j.nanoen.2021.106120.
Guo Q, Zheng Z. Rational design of binders for stable Li-S and Na-S batteries. Adv Funct Mater. 2020. https://doi.org/10.1002/adfm.201907931.
Yamamoto M, Goto S, Tang R, et al. Nano-confinement of insulating sulfur in the cathode composite of all-solid-state Li–S batteries using flexible carbon materials with large pore volumes. ACS Appl Mater Interfaces. 2021. https://doi.org/10.1021/acsami.1c10275.
Wang W, Wang D, Wang G, Zheng M, Wang G. Elastic, conductive coating layer for self-standing sulfur cathode achieving long lifespan Li–S batteries. Adv Energy Mater. 2020. https://doi.org/10.1002/aenm.201904026.
Zhao M, Li BQ, Peng HJ, Yuan H, Wei JY, Huang JQ. Lithium–sulfur batteries under lean electrolyte conditions: challenges and opportunities. Angew Chem Int Ed. 2020. https://doi.org/10.1002/anie.201909339.
Bhargav A, He J, Gupta A, Manthiram A. Lithium-sulfur batteries: attaining the critical metrics. Joule. 2020. https://doi.org/10.1016/j.joule.2019.12.009.
Yang Y, Wang W, Li L, Li B, Zhang J. Stable cycling of Li–S batteries by simultaneously suppressing Li-dendrite growth and polysulfide shuttling enabled by a bioinspired separator. J Mater Chem A. 2020. https://doi.org/10.1039/C9TA12921A.
Zhang R, Wu M, Fan X, Jiang H, Zhao T. A Li-S battery with ultrahigh cycling stability and enhanced rate capability based on novel ZnO yolk-shell sulfur host. J Energy Chem. 2021. https://doi.org/10.1016/j.jechem.2020.06.039.
Hou L-P, Zhang X-Q, Li B-Q, Zhang Q. Challenges and promises of lithium metal anode by soluble polysulfides in practical lithium–sulfur batteries. Mater Today. 2021. https://doi.org/10.1016/j.mattod.2020.10.021.
Cheng X-B, Yan C, Huang J-Q, et al. The gap between long lifespan Li-S coin and pouch cells: The importance of lithium metal anode protection. Energy Storage Mater. 2017. https://doi.org/10.1016/j.ensm.2016.09.003.
Sun X, Zhang X, Ma Q, Guan X, Wang W, Luo J. Revisiting the electroplating process for lithium-metal anodes for lithium-metal batteries. Angew Chem Int Ed. 2020. https://doi.org/10.1002/anie.201912217.
Mao H, Yu W, Cai Z, et al. Current-density regulating lithium metal directional deposition for long cycle-life Li metal batteries. Angew Chem. 2021. https://doi.org/10.1002/ange.202105831.
Gao R, Zhang M, Han Z, et al. Unraveling the coupling effect between cathode and anode toward practical lithium-sulfur batteries. Adv Mater. 2024. https://doi.org/10.1002/adma.202303610.
Cha E, Patel M, Bhoyate S, Prasad V, Choi W. Nanoengineering to achieve high efficiency practical lithium–sulfur batteries. Nanoscale Horiz. 2020. https://doi.org/10.1039/C9NH00730J.
Lee J, Moon JH. Polyhedral TiO2 particle-based cathode for Li-S batteries with high volumetric capacity and high performance in lean electrolyte. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2020.125670.
Feng Y, Wang G, Ju J, et al. Towards high energy density Li–S batteries with high sulfur loading: from key issues to advanced strategies. Energy Storage Mater. 2020. https://doi.org/10.1016/j.ensm.2020.06.043.
Zerrin T, Shang R, Dong B, et al. An overlooked parameter in Li-S batteries: the impact of electrolyte-to-sulfur ratio on capacity fading. Nano Energy. 2022. https://doi.org/10.1016/j.nanoen.2022.107913.
Zhu R, Yang H, Fadillah L, et al. A lithiophilic carbon scroll as a Li metal host with low tortuosity design and “Dead Li” self-cleaning capability. J Mater Chem A. 2021. https://doi.org/10.1039/D1TA02491D.
Luo Y, Guo L, **ao M, et al. Strategies for inhibiting anode dendrite growth in lithium–sulfur batteries. J Mater Chem A. 2020. https://doi.org/10.1039/C9TA12910C.
Shi Z, Li M, Sun J, Chen Z. Defect engineering for expediting Li–S chemistry: strategies, mechanisms, and perspectives. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202100332.
Yang D, Zhi R, Ruan D, et al. A multifunctional separator for high-performance lithium-sulfur batteries. Electrochim Acta. 2020. https://doi.org/10.1016/j.electacta.2019.135486.
He Y, Chang Z, Wu S, Zhou H. Effective strategies for long-cycle life lithium–sulfur batteries. J Mater Chem A. 2018. https://doi.org/10.1039/C8TA01115J.
Manthiram A, Fu Y, Chung S-H, Zu C, Su Y-S. Rechargeable lithium–sulfur batteries. Chem Rev. 2014. https://doi.org/10.1021/cr500062v.
He B, Rao Z, Cheng Z, et al. Rationally design a sulfur cathode with solid-phase conversion mechanism for high cycle-stable Li–S batteries. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202003690.
Zhang SS. Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions. J Power Sourc. 2013. https://doi.org/10.1016/j.jpowsour.2012.12.102.
Zheng J, Fan X, Ji G, et al. Manipulating electrolyte and solid electrolyte interphase to enable safe and efficient Li-S batteries. Nano Energy. 2018. https://doi.org/10.1016/j.nanoen.2018.05.065.
Yang Y, Zheng G, Misra S, Nelson J, Toney MF, Cui Y. High-capacity micrometer-sized Li2S particles as cathode materials for advanced rechargeable lithium-ion batteries. J Am Chem Soc. 2012. https://doi.org/10.1021/ja3052206.
Chen K-H, Wood KN, Kazyak E, et al. Dead lithium: mass transport effects on voltage, capacity, and failure of lithium metal anodes. J Mater Chem A. 2017. https://doi.org/10.1039/C7TA00371D.
Fang C, Li J, Zhang M, et al. Quantifying inactive lithium in lithium metal batteries. Nature. 2019. https://doi.org/10.1038/s41586-019-1481-z.
Chen X-R, Yan C, Ding J-F, Peng H-J, Zhang Q. New insights into “dead lithium” during strip** in lithium metal batteries. J Energy Chem. 2021. https://doi.org/10.1016/j.jechem.2021.03.048.
Dornbusch DA, Hilton R, Lohman SD, Suppes GJ. Experimental validation of the elimination of dendrite short-circuit failure in secondary lithium-metal convection cell batteries. J Electrochem Soc. 2014. https://doi.org/10.1149/2.0021503jes.
Chazalviel J-N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys Rev A. 1990. https://doi.org/10.1103/PhysRevA.42.7355.
Sand HJ. On the concentration at the electrodes in a solution, with special reference to the liberation of hydrogen by electrolysis of a mixture of copper sulphate and sulphuric acid. Proc Phys Soc. 1899. https://doi.org/10.1088/1478-7814/17/1/332.
Yoshimatsu I, Hirai T, Yamaki JI. Lithium electrode morphology during cycling in lithium cells. J Electrochem Soc. 1988. https://doi.org/10.1149/1.2095351.
Lu D, Shao Y, Lozano T, et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv Energy Mater. 2015. https://doi.org/10.1002/aenm.201400993.
Jiang FN, Yang SJ, Liu H, et al. Mechanism understanding for strip** electrochemistry of Li metal anode. SusMat. 2021. https://doi.org/10.1002/sus2.37.
Park J, Jeong J, Lee Y, Oh M, Ryou MH, Lee YM. Micro-patterned lithium metal anodes with suppressed dendrite formation for post lithium-ion batteries. Adv Mater Interfaces. 2016. https://doi.org/10.1002/admi.201600140.
Li Y, Jiao J, Bi J, Wang X, Wang Z, Chen L. Controlled deposition of Li metal. Nano Energy. 2017. https://doi.org/10.1016/j.nanoen.2016.12.030.
Seo J, Jeong W, Lim M, et al. Electrodeposition-guided pre-passivation of Li-metal anode to enable long stable cycling of practical Li-metal batteries. Energy Storage Mater. 2023. https://doi.org/10.1016/j.ensm.2023.102827.
Um JH, Yu SH. Unraveling the mechanisms of lithium metal plating/strip** via in situ/operando analytical techniques. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202003004.
Dey A. Electrochemical alloying of lithium in organic electrolytes. J Electrochem Soc. 1971. https://doi.org/10.1149/1.2407783.
Peled E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J Electrochem Soc. 1979. https://doi.org/10.1149/1.2128859.
Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater. 2010. https://doi.org/10.1021/cm901452z.
Jozwiuk A, Berkes BB, Weiß T, Sommer H, Janek J, Brezesinski T. The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries. Energy Environ Sci. 2016. https://doi.org/10.1039/C6EE00789A.
Schneider H, Weiß T, Scordilis-Kelley C, et al. Electrolyte decomposition and gas evolution in a lithium-sulfur cell upon long-term cycling. Electrochim Acta. 2017. https://doi.org/10.1016/j.electacta.2017.05.034.
Lin D, Liu Y, Li Y, et al. Fast galvanic lithium corrosion involving a Kirkendall-type mechanism. Nat Chem. 2019. https://doi.org/10.1038/s41557-018-0203-8.
Kolesnikov A, Kolek M, Dohmann JF, et al. Galvanic corrosion of lithium-powder-based electrodes. Adv Energy Mater. 2020. https://doi.org/10.1002/aenm.202000017.
Boyle DT, Huang W, Wang H, et al. Corrosion of lithium metal anodes during calendar ageing and its microscopic origins. Nat Energy. 2021. https://doi.org/10.1038/s41560-021-00787-9.
Jayaprakash N, Shen J, Moganty SS, Corona A, Archer LA. Porous hollow carbon@ sulfur composites for high-power lithium–sulfur batteries. Angew Chem Int Ed. 2011. https://doi.org/10.1002/anie.201100637.
Zheng D, Yang XQ, Qu D. Reaction between lithium anode and polysulfide ions in a lithium–sulfur battery. Chemsuschem. 2016. https://doi.org/10.1002/cssc.201600878.
Wang Q, ** J, Wu X, Ma G, Yang J, Wen Z. A shuttle effect free lithium sulfur battery based on a hybrid electrolyte. Phys Chem Chem Phys. 2014. https://doi.org/10.1039/C4CP03694H.
Yao YX, Zhang XQ, Li BQ, et al. A compact inorganic layer for robust anode protection in lithium-sulfur batteries. InfoMat. 2020. https://doi.org/10.1002/inf2.12046.
Camacho-Forero LE, Smith TW, Bertolini S, Balbuena PB. Reactivity at the lithium–metal anode surface of lithium–sulfur batteries. J Phys Chem C. 2015. https://doi.org/10.1021/acs.jpcc.5b08254.
Kim BDH, Lee MB, Park KY, Kang K. First-principles study on the charge transport mechanism of lithium sulfide (Li2S) in lithium-sulfur batteries. Chem Asian J. 2016. https://doi.org/10.1002/asia.201600007.
Huang F, Wang S, Jie Y, et al. Deciphering pitting behavior of lithium metal anodes in lithium sulfur batteries. J Energy Chem. 2020. https://doi.org/10.1016/j.jechem.2020.02.039.
Zhang Y, Heim FM, Song N, Bartlett JL, Li X. New insights into mossy Li induced anode degradation and its formation mechanism in Li–S batteries. ACS Energy Lett. 2017. https://doi.org/10.1021/acsenergylett.7b00886.
Sun M, Wang X, Wang J, Yang H, Wang L, Liu T. Assessment on the self-discharge behavior of lithium–sulfur batteries with LiNO3-possessing electrolytes. ACS Appl Mater Interfaces. 2018. https://doi.org/10.1021/acsami.8b11890.
Ye H, Li Y. Room-temperature metal–sulfur batteries: What can we learn from lithium–sulfur? InfoMat. 2022. https://doi.org/10.1002/inf2.12291.
Ye H, Lee JY. Solid additives for improving the performance of sulfur cathodes in lithium–sulfur batteries—adsorbents, mediators, and catalysts. Small Methods. 2020. https://doi.org/10.1002/smtd.201900864.
Wu J, Wu Y, Wang L, Ye H, Lu J, Li Y. Challenges and advances in rechargeable batteries for extreme-condition applications. Adv Mater. 2024. https://doi.org/10.1002/adma.202308193.
Yan C, Cheng X-B, Zhao C-Z, Huang J-Q, Yang S-T, Zhang Q. Lithium metal protection through in-situ formed solid electrolyte interphase in lithium-sulfur batteries: The role of polysulfides on lithium anode. J Power Sourc. 2016. https://doi.org/10.1016/j.jpowsour.2016.07.056.
Zhang Y, Liu S, Wang X, et al. Composite Li metal anode with vertical graphene host for high performance Li-S batteries. J Power Sourc. 2018. https://doi.org/10.1016/j.jpowsour.2017.10.057.
Shen X, Qian T, Chen P, Liu J, Wang M, Yan C. Bioinspired polysulfiphobic artificial interphase layer on lithium metal anodes for lithium sulfur batteries. ACS Appl Mater Interfaces. 2018. https://doi.org/10.1021/acsami.8b12093.
Cui C, Zhang R, Fu C, et al. Stabilizing lithium metal anode enabled by a natural polymer layer for lithium-sulfur batteries. ACS Appl Mater Interfaces. 2021. https://doi.org/10.1021/acsami.1c06289.
Xu R, Cheng X-B, Yan C, et al. Artificial Interphases for highly stable lithium metal anode. Matter. 2019. https://doi.org/10.1016/j.matt.2019.05.016.
Chen XR, Yao YX, Yan C, Zhang R, Cheng XB, Zhang Q. A diffusion–reaction competition mechanism to tailor lithium deposition for lithium-metal batteries. Angew Chem Int Ed. 2020. https://doi.org/10.1002/anie.202000375.
Liu Y, Xu X, Kapitanova OO, et al. Electro-chemo-mechanical modeling of artificial solid electrolyte interphase to enable uniform electrodeposition of lithium metal anodes. Adv Energy Mater. 2022. https://doi.org/10.1002/aenm.202103589.
Liu W, Liu P, Mitlin D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv Energy Mater. 2020. https://doi.org/10.1002/aenm.202002297.
Kang D, **ao M, Lemmon JP. Artificial solid-electrolyte interphase for lithium metal batteries. Batter Supercaps. 2021. https://doi.org/10.1002/batt.202000225.
Grotkopp NL, Horst M, Batzer M, Garnweitner G, Jean-Fulcrand A. Flexible freestanding thin polyethylene oxide-based film as artificial solid-electrolyte interface to protect lithium metal in lithium-sulfur batteries. Adv Energy Sustain Res. 2023. https://doi.org/10.1002/aesr.202200146.
Chen D, Huang S, Zhong L, et al. In situ preparation of thin and rigid COF film on Li anode as artificial solid electrolyte interphase layer resisting Li dendrite puncture. Adv Funct Mater. 2020. https://doi.org/10.1002/adfm.201907717.
Liu J, Cao Y, Zhou J, et al. Artificial lithium isopropyl-sulfide macromolecules as an ion-selective interface for long-life lithium–sulfur batteries. ACS Appl Mater Interfaces. 2020. https://doi.org/10.1021/acsami.0c13835.
Ma G, Wen Z, Wang Q, Shen C, ** J, Wu X. Enhanced cycle performance of a Li–S battery based on a protected lithium anode. J Mater Chem A. 2014. https://doi.org/10.1039/C4TA04172K.
Wu M, ** J, Wen Z. Influence of a surface modified Li anode on the electrochemical performance of Li–S batteries. RCS Adv. 2016. https://doi.org/10.1039/C6RA05316E.
Kim MS, Kim M-S, Do V, et al. Designing solid-electrolyte interphases for lithium sulfur electrodes using ionic shields. Nano Energy. 2017. https://doi.org/10.1016/j.nanoen.2017.10.018.
Kozen AC, Lin C-F, Pearse AJ, et al. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano. 2015. https://doi.org/10.1021/acsnano.5b02166.
Wang W, Yue X, Meng J, et al. Lithium phosphorus oxynitride as an efficient protective layer on lithium metal anodes for advanced lithium-sulfur batteries. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2018.08.010.
Luo L, Manthiram A. An artificial protective coating toward dendrite-free lithium-metal anodes for lithium-sulfur batteries. Energy Technol. 2020. https://doi.org/10.1002/ente.202000348.
**ong C, Ren Y, Jiang H, Wu M, Zhao T. Artificial bifunctional protective layer composed of carbon nitride nanosheets for high performance lithium–sulfur batteries. J Energy Storage. 2019. https://doi.org/10.1016/j.est.2019.101006.
Sun C, Huang X, ** J, et al. An ion-conductive Li1. 5Al0. 5Ge1. 5 (PO4) 3-based composite protective layer for lithium metal anode in lithium-sulfur batteries. J Power Sour. 2018. https://doi.org/10.1016/j.jpowsour.2017.11.063.
** Q, Zhang X, Gao H, Li L, Zhang Z. Novel Li x SiS y/Nafion as an artificial SEI film to enable dendrite-free Li metal anodes and high stability Li–S batteries. J Mater Chem A. 2020. https://doi.org/10.1039/D0TA02999H.
Lu W, Wang Z, Sun G, et al. Anchoring polysulfide with artificial solid electrolyte interphase for dendrite-free and low N/P ratio Li-S batteries. J Energy Chem. 2023. https://doi.org/10.1016/j.jechem.2023.01.032.
Wang H, Liu Y, Li Y, Cui Y. Lithium metal anode materials design: interphase and host. Electrochem Energy Rev. 2019. https://doi.org/10.1007/s41918-019-00054-2.
Cheng Y, Chen J, Chen Y, et al. Lithium host: advanced architecture components for lithium metal anode. Energy Storage Mater. 2021. https://doi.org/10.1016/j.ensm.2021.03.008.
Sand HJ III. On the concentration at the electrodes in a solution, with special reference to the liberation of hydrogen by electrolysis of a mixture of copper sulphate and sulphuric acid. Lond Edinb Dubl Phil Mag. 1901. https://doi.org/10.1080/14786440109462590.
Yue X-Y, Bao J, Qiu Q-Q, et al. Copper decorated ultralight 3D carbon skeleton derived from soybean oil for dendrite-free Li metal anode. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2019.123516.
Wang Q, Yang J, Huang X, et al. Rigid and flexible SEI layer formed over a cross-linked polymer for enhanced ultrathin Li metal anode performance. Adv Energy Mater. 2022. https://doi.org/10.1002/aenm.202103972.
Gu X, Dong J, Lai C. Li-containing alloys beneficial for stabilizing lithium anode: a review. Eng Rep. 2021. https://doi.org/10.1002/eng2.12339.
Hiratani M, Miyauchi K, Kudo T. Effect of a lithium alloy layer inserted between a lithium anode and a solid electrolyte. Solid State Ion. 1988. https://doi.org/10.1016/0167-2738(88)90394-3.
Corradini M, Jeppson D. Lithium alloy chemical reactivity with reactor materials: current state of knowledge. Fusion Eng Des. 1991. https://doi.org/10.1016/0920-3796(91)90011-E.
Kong LL, Wang L, Ni ZC, Liu S, Li GR, Gao XP. Lithium–magnesium alloy as a stable anode for lithium–sulfur battery. Adv Funct Mater. 2019. https://doi.org/10.1002/adfm.201808756.
**a S, Zhang X, Liang C, Yu Y, Liu W. Stabilized lithium metal anode by an efficient coating for high-performance Li–S batteries. Energy Storage Mater. 2020. https://doi.org/10.1016/j.ensm.2019.07.042.
Li R, Yang L, Song L, et al. A thin LiGa alloy layer from in-situ electroreduction to suppress anode dendrite formation in lithium-sulfur pouch cell. Chem Eng J. 2023. https://doi.org/10.1016/j.cej.2022.140707.
Lv S, Ma X, Ke S, et al. Metal-coordinated covalent organic frameworks as advanced bifunctional hosts for both sulfur cathodes and lithium anodes in lithium-sulfur batteries. J Am Chem Soc. 2024. https://doi.org/10.1021/jacs.4c01620.
Yao Y, Wang H, Yang H, et al. A dual-functional conductive framework embedded with TiN-VN heterostructures for highly efficient polysulfide and lithium regulation toward stable Li–S full batteries. Adv Mater. 2020. https://doi.org/10.1002/adma.201905658.
Wei Y, Wang B, Zhang Y, et al. Rational design of multifunctional integrated host configuration with lithiophilicity-sulfiphilicity toward high-performance Li–S full batteries. Adv Funct Mater. 2021. https://doi.org/10.1002/adfm.202006033.
Mikhaylik YV, Kovalev I, Schock R, Kumaresan K, Xu J, Affinito J. High energy rechargeable Li-S cells for EV application: status, remaining problems and solutions. ECS Trans. 2010. https://doi.org/10.1149/1.3414001.
Zhang S, Ueno K, Dokko K, Watanabe M. Recent advances in electrolytes for lithium–sulfur batteries. Adv Energy Mater. 2015. https://doi.org/10.1002/aenm.201500117.
Lacey MJ, Yalamanchili A, Maibach J, Tengstedt C, Edström K, Brandell D. The Li–S battery: an investigation of redox shuttle and self-discharge behaviour with LiNO 3-containing electrolytes. RCS Adv. 2016. https://doi.org/10.1039/C5RA23635E.
Zhang L, Ling M, Feng J, Mai L, Liu G, Guo J. The synergetic interaction between LiNO3 and lithium polysulfides for suppressing shuttle effect of lithium-sulfur batteries. Energy Storage Mater. 2018. https://doi.org/10.1016/j.ensm.2017.09.001.
Li S, Dai H, Li Y, et al. Designing Li-protective layer via SOCl2 additive for stabilizing lithium-sulfur battery. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2018.09.012.
Lian J, Guo W, Fu Y. Isomeric organodithiol additives for improving interfacial chemistry in rechargeable Li–S batteries. J Am Chem Soc. 2021. https://doi.org/10.1021/jacs.1c04222.
Ding F, Xu W, Graff GL, et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J Am Chem Soc. 2013. https://doi.org/10.1021/ja312241y.
Wei JY, Zhang XQ, Hou LP, et al. Shielding polysulfide intermediates by an organosulfur-containing solid electrolyte interphase on the lithium anode in lithium–sulfur batteries. Adv Mater. 2020. https://doi.org/10.1002/adma.202003012.
Li J, Liu S, Cui Y, et al. Potassium hexafluorophosphate additive enables stable lithium–sulfur batteries. ACS Appl Mater Interfaces. 2020. https://doi.org/10.1021/acsami.0c17406.
Wang Z, Li Y, Ji H, Zhou J, Qian T, Yan C. Unity of opposites between soluble and insoluble lithium polysulfides in lithium–sulfur batteries. Adv Mater. 2022. https://doi.org/10.1002/adma.202203699.
Schmeisser M, Illner P, Puchta R, Zahl A, van Eldik R. Gutmann donor and acceptor numbers for ionic liquids. Chem Eur J. 2012. https://doi.org/10.1002/chem.201200584.
Qu C, Chen Y, Yang X, Zhang H, Li X, Zhang H. LiNO3-free electrolyte for Li-S battery: a solvent of choice with low Ksp of polysulfide and low dendrite of lithium. Nano Energy. 2017. https://doi.org/10.1016/j.nanoen.2017.07.002.
Weller C, Thieme S, Härtel P, Althues H, Kaskel S. Intrinsic shuttle suppression in lithium-sulfur batteries for pouch cell application. J Electrochem Soc. 2017. https://doi.org/10.1149/2.0981714jes.
Sun K, Wu Q, Tong X, Gan H. Electrolyte with low polysulfide solubility for Li–S batteries. ACS Appl Energy Mater. 2018. https://doi.org/10.1021/acsaem.8b00317.
Pang Q, Shyamsunder A, Narayanan B, Kwok CY, Curtiss LA, Nazar LF. Tuning the electrolyte network structure to invoke quasi-solid state sulfur conversion and suppress lithium dendrite formation in Li–S batteries. Nat Energy. 2018. https://doi.org/10.1038/s41560-018-0214-0.
Weller C, Pampel J, Dörfler S, Althues H, Kaskel S. Polysulfide shuttle suppression by electrolytes with low-density for high-energy lithium–sulfur batteries. Energy Technol. 2019. https://doi.org/10.1002/ente.201900625.
Gordin ML, Dai F, Chen S, et al. Bis (2, 2, 2-trifluoroethyl) ether as an electrolyte co-solvent for mitigating self-discharge in lithium–sulfur batteries. ACS Appl Mater Interfaces. 2014. https://doi.org/10.1021/am501665s.
Sun K, Wu Q, Gan H. Molecular insights into ether-based electrolytes for Li-FeS2 batteries. Energy Storage Mater. 2018. https://doi.org/10.1016/j.ensm.2017.12.003.
Shin M, Wu H-L, Narayanan B, et al. Effect of the hydrofluoroether cosolvent structure in acetonitrile-based solvate electrolytes on the Li+ solvation structure and Li–S battery performance. ACS Appl Mater Interfaces. 2017. https://doi.org/10.1021/acsami.7b11566.
Azimi N, Xue Z, Bloom I, et al. Understanding the effect of a fluorinated ether on the performance of lithium–sulfur batteries. ACS Appl Mater Interfaces. 2015. https://doi.org/10.1021/acsami.5b01412.
Drvarič Talian S, Jeschke S, Vizintin A, et al. Fluorinated ether based electrolyte for high-energy lithium-sulfur batteries: Li+ solvation role behind reduced polysulfide solubility. Chem Mater. 2017. https://doi.org/10.1021/acs.chemmater.7b03654.
Su CC, He M, Amine R, Amine K. A selection rule for hydrofluoroether electrolyte cosolvent: establishing a linear free-energy relationship in lithium–sulfur batteries. Angew Chem. 2019. https://doi.org/10.1002/ange.201904240.
Qian J, Henderson WA, Xu W, et al. High rate and stable cycling of lithium metal anode. Nat Commun. 2015. https://doi.org/10.1038/ncomms7362.
Suo L, Hu Y-S, Li H, Armand M, Chen L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat Commun. 2013. https://doi.org/10.1038/ncomms2513.
Cuisinier M, Cabelguen P-E, Adams B, Garsuch A, Balasubramanian M, Nazar L. Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries. Energy Environ Sci. 2014. https://doi.org/10.1039/C4EE00372A.
Jiang Z, Zeng Z, Hu W, Han Z, Cheng S, **e J. Diluted high concentration electrolyte with dual effects for practical lithium-sulfur batteries. Energy Storage Mater. 2021. https://doi.org/10.1016/j.ensm.2021.01.008.
Zhang XQ, ** Q, Nan YL, et al. Electrolyte structure of lithium polysulfides with anti-reductive solvent shells for practical lithium–sulfur batteries. Angew Chem Int Ed. 2021. https://doi.org/10.1002/anie.202103470.
Hou LP, Li Z, Yao N, et al. Weakening the solvating power of solvents to encapsulate lithium polysulfides enables long-cycling lithium–sulfur batteries. Adv Mater. 2022. https://doi.org/10.1002/adma.202205284.
Hou L-P, Zhang X-Q, Yao N, et al. An encapsulating lithium-polysulfide electrolyte for practical lithium–sulfur batteries. Chem. 2022. https://doi.org/10.1016/j.chempr.2021.12.023.
Ueno K, Park J-W, Yamazaki A, et al. Anionic effects on solvate ionic liquid electrolytes in rechargeable lithium-sulfur batteries. J Phys Chem C. 2013. https://doi.org/10.1021/jp407158y.
Meisner QJ, Rojas T, Glossmann T, et al. Impact of co-solvent and LiTFSI Concentration on ionic liquid-based electrolytes for Li-S battery. J Electrochem Soc. 2020. https://doi.org/10.1149/1945-7111/ab76a3.
Barghamadi M, Best AS, Bhatt AI, et al. Lithium–sulfur batteries—the solution is in the electrolyte, but is the electrolyte a solution? Energy Environ Sci. 2014. https://doi.org/10.1039/C4EE02192D.
Umeshbabu E, Zheng B, Yang Y. Recent progress in all-solid-state Lithium−sulfur batteries using high Li-ion conductive solid electrolytes. Electrochem Energy Rev. 2019. https://doi.org/10.1007/s41918-019-00029-3.
Yang X, Luo J, Sun X. Towards high-performance solid-state Li–S batteries: from fundamental understanding to engineering design. Chem Soc Rev. 2020. https://doi.org/10.1039/C9CS00635D.
Wang D, Jhang L-J, Kou R, et al. Realizing high-capacity all-solid-state lithium-sulfur batteries using a low-density inorganic solid-state electrolyte. Nat Commun. 2023. https://doi.org/10.1038/s41467-023-37564-z.
Wang J, Yang J, Wan C, Du K, **e J, Xu N. Sulfur composite cathode materials for rechargeable lithium batteries. Adv Funct Mater. 2003. https://doi.org/10.1002/adfm.200304284.
Nagata H, Chikusa Y. Activation of sulfur active material in an all-solid-state lithium–sulfur battery. J Power Sour. 2014. https://doi.org/10.1016/j.jpowsour.2014.04.032.
Murugan R, Thangadurai V, Weppner W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed Engl. 2007. https://doi.org/10.1002/anie.200701144.
Han X, Gong Y, Fu K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat Mater. 2017. https://doi.org/10.1038/nmat4821.
Wang Q, Ding X, Li J, ** H, Gao H. Minimizing the interfacial resistance for a solid-state lithium battery running at room temperature. Chem Eng J. 2022. https://doi.org/10.1016/j.cej.2022.137740.
Wei R, Chen S, Gao T, Liu W. Challenges, fabrications and horizons of oxide solid electrolytes for solid-state lithium batteries. Nano Sel. 2021. https://doi.org/10.1002/nano.202100110.
Song Y-X, Shi Y, Wan J, et al. Direct tracking of the polysulfide shuttling and interfacial evolution in all-solid-state lithium–sulfur batteries: a degradation mechanism study. Energy Environ Sci. 2019. https://doi.org/10.1039/C9EE00578A.
Li X, Wang D, Wang H, Yan H, Gong Z, Yang Y. Poly (ethylene oxide)–Li10SnP2S12 composite polymer electrolyte enables high-performance all-solid-state lithium sulfur battery. ACS Appl Mater Interfaces. 2019. https://doi.org/10.1021/acsami.9b05212.
Liu Y, Xu B, Zhang W, Li L, Lin Y, Nan C. Composition modulation and structure design of inorganic-in-polymer composite solid electrolytes for advanced lithium batteries. Small. 2020. https://doi.org/10.1002/smll.201902813.
Zhang X, Zhang H, Geng Y, et al. A multifunctional nano filler for solid polymer electrolyte toward stable cycling for lithium-metal anodes in lithium–sulfur batteries. Chem Eng J. 2022. https://doi.org/10.1016/j.cej.2022.136328.
Fang R, Xu H, Xu B, Li X, Li Y, Goodenough JB. Reaction mechanism optimization of solid-state Li–S batteries with a PEO-based electrolyte. Adv Funct Mater. 2021. https://doi.org/10.1002/adfm.202001812.
Pope MA, Aksay IA. Structural design of cathodes for Li-S batteries. Adv Energy Mater. 2015. https://doi.org/10.1002/aenm.201500124.
Ryu HS, Park JW, Park J, et al. High capacity cathode materials for Li–S batteries. J Mater Chem A. 2013. https://doi.org/10.1039/C2TA00056C.
Lim W-G, Mun Y, Cho A, et al. Synergistic effect of molecular-type electrocatalysts with ultrahigh pore volume carbon microspheres for lithium–sulfur batteries. ACS Nano. 2018. https://doi.org/10.1021/acsnano.8b02258.
Ji X, Lee KT, Nazar LF. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat Mater. 2009. https://doi.org/10.1038/nmat2460.
Yang L, Li Q, Wang Y, et al. A review of cathode materials in lithium-sulfur batteries. Ionics. 2020. https://doi.org/10.1007/s11581-020-03767-3.
Yu G, Ye G, Wang C, et al. A flame-retardant binder with high polysulfide affinity for safe and stable lithium-sulfur batteries. Sci China Chem. 2024. https://doi.org/10.1007/s11426-023-1808-2.
Yu G, Wang C-Y, Dong W, et al. Anion-doped polypyrrole three-dimensional framework enables adsorption and conversion in lithium–sulfur batteries. J Colloid Interface Sci. 2024. https://doi.org/10.1016/j.jcis.2023.10.033.
Yan M, Wang ZY, Yu GW, et al. Adsorption-catalysis-conversion of polysulfides in sandwiched ultrathin Ni (OH) 2-PANI for stable lithium-sulfur batteries. Small. 2022. https://doi.org/10.1002/smll.202201822.
Liang Q, Wang S, Yao Y, Dong P, Song H. Transition metal compounds family for Li–S batteries: the DFT-guide for suppressing polysulfides shuttle. Adv Funct Mater. 2023. https://doi.org/10.1002/adfm.202300825.
Li Y, Zhang J, Chen Q, **a X, Chen M. Emerging of heterostructure materials in energy storage: a review. Adv Mater. 2021. https://doi.org/10.1002/adma.202100855.
Zhuang Z, Kang Q, Wang D, Li Y. Single-atom catalysis enables long-life, high-energy lithium-sulfur batteries. Nano Res. 2020. https://doi.org/10.1007/s12274-020-2827-4.
Liu X, Huang JQ, Zhang Q, Mai L. Nanostructured metal oxides and sulfides for lithium–sulfur batteries. Adv Mater. 2017. https://doi.org/10.1002/adma.201601759.
Li J, Niu Z, Guo C, Li M, Bao W. Catalyzing the polysulfide conversion for promoting lithium sulfur battery performances: a review. J Energy Chem. 2021. https://doi.org/10.1016/j.jechem.2020.06.009.
Ng SF, Lau MYL, Ong WJ. Lithium–sulfur battery cathode design: tailoring metal-based nanostructures for robust polysulfide adsorption and catalytic conversion. Adv Mater. 2021. https://doi.org/10.1002/adma.202008654.
Wang H, Zhang W, Xu J, Guo Z. Advances in polar materials for lithium–sulfur batteries. Adv Funct Mater. 2018. https://doi.org/10.1002/adfm.201707520.
Deng S, Guo T, Heier J, Zhang C. Unraveling polysulfide’s adsorption and electrocatalytic conversion on metal oxides for Li-S batteries. Adv Sci. 2023. https://doi.org/10.1002/advs.202204930.
Wang J, Li G, Luo D, et al. Engineering the conductive network of metal oxide-based sulfur cathode toward efficient and longevous lithium–sulfur batteries. Adv Energy Mater. 2020. https://doi.org/10.1002/aenm.202002076.
Zheng Y, Yi Y, Fan M, et al. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2019.02.030.
Yan B, Li X, **ao W, Hu J, Zhang L, Yang X. Design, synthesis, and application of metal sulfides for Li–S batteries: progress and prospects. J Mater Chem A. 2020. https://doi.org/10.1039/D0TA06220K.
Wu J, Ye T, Wang Y, et al. Understanding the catalytic kinetics of polysulfide redox reactions on transition metal compounds in Li–S batteries. ACS Nano. 2022. https://doi.org/10.1021/acsnano.2c08581.
Kim SJ, Kim K, Park J, Sung YE. Role and potential of metal sulfide catalysts in Lithium-Sulfur battery applications. ChemCatChem. 2019. https://doi.org/10.1002/cctc.201900184.
Chen X, Peng H-J, Zhang R, et al. An analogous periodic law for strong anchoring of polysulfides on polar hosts in lithium sulfur batteries: S-or Li-binding on first-row transition-metal sulfides? ACS Energy Lett. 2017. https://doi.org/10.1021/acsenergylett.7b00164.
Ren Y, Yu C, Song X, et al. A tuned Lewis acidic catalyst guided by hard–soft acid–base theory to promote N 2 electroreduction. J Mater Chem A. 2021. https://doi.org/10.1039/D1TA02681J.
Zhang CY, Sun GW, Liu QY, et al. Deciphering the catalysis essence of vanadium self-intercalated two-dimensional vanadium sulfides (V5S8) on lithium polysulfide towards high-rate and ultra-stable Li-S batteries. Energy Storage Mater. 2021. https://doi.org/10.1016/j.ensm.2021.09.030.
Yu J, **ao J, Li A, et al. Enhanced multiple anchoring and catalytic conversion of polysulfides by amorphous MoS3 nanoboxes for high-performance Li-S batteries. Angew Chem Int Ed. 2020. https://doi.org/10.1002/anie.202004914.
Shao Q, Lu P, Xu L, et al. Rational design of MoS2 nanosheets decorated on mesoporous hollow carbon spheres as a dual-functional accelerator in sulfur cathode for advanced pouch-type Li–S batteries. J Energy Chem. 2020. https://doi.org/10.1016/j.jechem.2020.03.035.
Zhang Y, Zhang P, Li B, et al. Vertically aligned graphene nanosheets on multi-yolk/shell structured TiC@ C nanofibers for stable Li–S batteries. Energy Storage Mater. 2020. https://doi.org/10.1016/j.ensm.2020.01.029.
Hong X-J, Song C-L, Wu Z-M, et al. Sulfophilic and lithophilic sites in bimetal nickel-zinc carbide with fast conversion of polysulfides for high-rate Li-S battery. Chem Eng J. 2021. https://doi.org/10.1016/j.cej.2020.126566.
Liu Z, Lian R, Wu Z, et al. Ordered dual-channel carbon embedded with molybdenum nitride catalytically induced high-performance Lithium-Sulfur battery. Chem Eng J. 2022. https://doi.org/10.1016/j.cej.2021.134163.
Yang X, Chen S, Gong W, et al. Kinetic enhancement of sulfur cathodes by N-doped porous graphitic carbon with bound VN nanocrystals. Small. 2020. https://doi.org/10.1002/smll.202004950.
Wang Y, Wei Y, Wang B, et al. Bio-assisted engineering of hierarchical porous carbon nanofiber host in-situ embedded with iron carbide nanocatalysts toward high-performance Li–S batteries. Carbon. 2021. https://doi.org/10.1016/j.carbon.2021.02.073.
Mao Y, Sun W, Yue X, et al. Flexible free-standing cathode enabled via multifunctional Mo2C for high sulfur loading lithium–sulfur batteries. J Power Sour. 2021. https://doi.org/10.1016/j.jpowsour.2021.230254.
Yan Y, Li H, Cheng C, et al. Boosting polysulfide redox conversion of Li-S batteries by one-step-synthesized Co-Mo bimetallic nitride. J Energy Chem. 2021. https://doi.org/10.1016/j.jechem.2021.03.041.
Lim WG, Jo C, Cho A, et al. Approaching ultrastable high-rate Li–S batteries through hierarchically porous titanium nitride synthesized by multiscale phase separation. Adv Mater. 2019. https://doi.org/10.1002/adma.201806547.
Huang S, Wang Z, Von Lim Y, et al. Recent advances in heterostructure engineering for lithium–sulfur batteries. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202003689.
Hou R, Zhang S, Zhang P, et al. Ti 3 C 2 MXene as an “energy band bridge” to regulate the heterointerface mass transfer and electron reversible exchange process for Li–S batteries. J Mater Chem A. 2020. https://doi.org/10.1039/d0ta06695h.
Wang H, Wei Y, Wang G, et al. Selective nitridation crafted a high-density, carbon-free heterostructure host with built-in electric field for enhanced energy density Li–S batteries. Adv Sci. 2022. https://doi.org/10.1002/advs.202201823.
Bai Y, Nguyen TT, Chu R, Song H, Kim NH, Lee JH. Heterostructured TiN/TiO2 on the hierarchical N-doped carbon for enhancing the polysulfide immobilization and sulfur reduction in lithium-sulfur battery. Chem Eng J. 2023. https://doi.org/10.1016/j.cej.2023.146581.
He J, Manthiram A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2019.04.038.
Zhang L, Liu Y, Zhao Z, et al. Enhanced polysulfide regulation via porous catalytic V2O3/V8C7 heterostructures derived from metal–organic frameworks toward high-performance Li–S batteries. ACS Nano. 2020. https://doi.org/10.1021/acsnano.0c02762.
**ong W, Lin J, Wang H, et al. Construction of strong built-in electric field in binary metal sulfide heterojunction to propel high-loading lithium-sulfur batteries. J Energy Chem. 2023. https://doi.org/10.1016/j.jechem.2023.03.012.
Wang Y, Chu F, Zeng J, et al. Single atom catalysts for fuel cells and rechargeable batteries: principles, advances, and opportunities. ACS Nano. 2021. https://doi.org/10.1021/acsnano.0c08652.
Zhou T, Liang J, Ye S, Zhang Q, Liu J. Fundamental, application and opportunities of single atom catalysts for Li-S batteries. Energy Storage Mater. 2023. https://doi.org/10.1002/adma.202200102.
Liang Z, Shen J, Xu X, et al. Advances in the development of single-atom catalysts for high-energy-density lithium–sulfur batteries. Adv Mater. 2022. https://doi.org/10.1002/adma.202200102.
Lim WG, Park CY, Jung H, et al. Cooperative electronic structure modulator of Fe single-atom electrocatalyst for high energy and long cycle Li–S pouch cell. Adv Mater. 2023. https://doi.org/10.1002/adma.202208999.
Liu K, Wang X, Gu S, et al. N, S-coordinated co single atomic catalyst boosting adsorption and conversion of Lithium polysulfides for Lithium-Sulfur batteries. Small. 2022. https://doi.org/10.1002/smll.202204707.
Zhang Y, Kang C, Zhao W, et al. dp hybridization-induced “Trap**–Coupling–Conversion” enables high-efficiency Nb single-atom catalysis for Li–S batteries. J Am Chem Soc. 2023. https://doi.org/10.1021/jacs.2c10345.
Son D, Lim W-G, Lee J. A short review of the recent developments in functional separators for lithium-sulfur batteries. Korean J Chem Eng. 2023. https://doi.org/10.1007/s11814-022-1372-0.
Son D, Park H, Lim W-G, et al. Ultrathin mixed ionic-electronic conducting interlayer via the solution shearing technique for high-performance Lithium-Sulfur batteries. ACS Nano. 2023. https://doi.org/10.1021/acsnano.3c09333.
Fan X, Liu Y, Tan J, et al. An ultrathin and highly efficient interlayer for lithium–sulfur batteries with high sulfur loading and lean electrolyte. J Mater Chem A. 2022. https://doi.org/10.1039/d1ta10444f.
Kim S, Lim W-G, Im H, et al. Polymer interface-dependent morphological transition toward two-dimensional porous inorganic nanocoins as an ultrathin multifunctional layer for stable lithium–sulfur batteries. J Am Chem Soc. 2021. https://doi.org/10.1021/jacs.1c05562.
Jiang G, Zheng N, Chen X, et al. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem Eng J. 2019. https://doi.org/10.1016/j.cej.2019.05.119.
Zhang M, Gui AL, Sun W, et al. High capacity utilization of Li metal anodes by application of celgard separator-reinforced ternary polymer electrolyte. J Electrochem Soc. 2019. https://doi.org/10.1149/2.1131910jes.
Hu C, Chen H, Shen Y, et al. In situ wrap** of the cathode material in lithium-sulfur batteries. Nat Commun. 2017. https://doi.org/10.1038/s41467-017-00656-8.
Abbas SA, Ding J, Wu SH, et al. Modified separator performing dual physical/chemical roles to inhibit polysulfide shuttle resulting in ultrastable Li–S batteries. ACS Nano. 2017. https://doi.org/10.1021/acsnano.7b06478.
Deng N, Kang W, Liu Y, et al. A review on separators for lithiumsulfur battery: progress and prospects. J Power Sour. 2016. https://doi.org/10.1016/j.jpowsour.2016.09.044.
Aslam MK, Jamil S, Hussain S, Xu M. Effects of catalysis and separator functionalization on high-energy lithium–sulfur batteries: a complete review. Energy Environ Mater. 2023. https://doi.org/10.1002/eem2.12420.
Kong Y, Wang L, Mamoor M, et al. Co/Mon invigorated bilateral kinetics modulation for advanced Lithium-Sulfur batteries. Adv Mater. 2023. https://doi.org/10.1002/adma.202310143.
Zou K, Chen X, **g W, et al. Facilitating catalytic activity of indium oxide in lithium-sulfur batteries by controlling oxygen vacancies. Energy Storage Mater. 2022. https://doi.org/10.1016/j.ensm.2022.03.003.
Liu J, Qiao Z, **e Q, Peng D-L, **e R-J. Phosphorus-doped metal-organic framework-derived CoS2 nanoboxes with improved adsorption-catalysis effect for Li–S batteries. ACS Appl Mater Interfaces. 2021. https://doi.org/10.1021/acsami.1c00494.
Kim S, Lim W-G, Cho A, et al. Simultaneous suppression of shuttle effect and lithium dendrite growth by lightweight bifunctional separator for Li–S batteries. ACS Appl Energy Mater. 2020. https://doi.org/10.1021/acsaem.9b02350.
Wang M, Ruan G, Gao Q, et al. Zn2+–Modulated bimetallic carbides synergized with macro-mesoporous N-rich carbon enabling accelerated polysulfides conversion for high-performance Li-S batteries. Chem Eng J. 2022. https://doi.org/10.1016/j.cej.2021.133205.
Lv Y, Bai L, ** Q, et al. VSe2/V2C heterocatalyst with built-in electric field for efficient lithium-sulfur batteries: Remedies polysulfide shuttle and conversion kinetics. J Energy Chem. 2024. https://doi.org/10.1016/j.jechem.2023.10.003.
**g W, Tan Q, Duan Y, et al. Defect-rich single atom catalyst enhanced polysulfide conversion kinetics to upgrade performance of Li–S batteries. Small. 2023. https://doi.org/10.1002/smll.202204880.
Zhang Y, Ma C, Zhang C, et al. Selective catalysis of single V atoms and VN1-x nanodots enables fast polysulfides conversion in lithium–sulfur batteries. Chem Eng J. 2023. https://doi.org/10.1016/j.cej.2022.139410.
Liu H, Cheng X-B, Huang J-Q, et al. Controlling dendrite growth in solid-state electrolytes. ACS Energy Lett. 2020. https://doi.org/10.1021/acsenergylett.9b02660.
Wang C, Wang A, Ren L, et al. Controlling Li ion flux through materials innovation for dendrite-free lithium metal anodes. Adv Funct Mater. 2019. https://doi.org/10.1002/adfm.201905940.
Wang Z, Huang W, Hua J, et al. An anionic-MOF-based bifunctional separator for regulating lithium deposition and suppressing polysulfides shuttle in Li–S batteries. Small Methods. 2020. https://doi.org/10.1002/smtd.202000082.
Li Y, Wang W, Liu X, et al. Engineering stable electrode-separator interfaces with ultrathin conductive polymer layer for high-energy-density Li-S batteries. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2019.05.005.
An Q, Wang L, Zhao G, et al. Constructing cooperative interface via Bi-functional COF for facilitating the sulfur conversion and Li+ dynamics. Adv Mater. 2024. https://doi.org/10.1002/adma.202305818.
Lim W-G, Oh S, Jeong J, et al. Ultrathin and bifunctional polymer-nanolayer-embedded separator to simultaneously alleviate Li dendrite growth and polysulfide crossover in Li–S batteries. ACS Appl Energy Mater. 2021. https://doi.org/10.1021/acsaem.0c00970.
Fan C, Yang R, Huang Y, et al. Graphene quantum dots as sulfiphilic and lithiophilic mediator toward high stability and durable life lithium-sulfur batteries. J Energy Chem. 2023. https://doi.org/10.1016/j.jechem.2023.06.030.
Yan W, Yang JL, **ong X, et al. Versatile asymmetric separator with dendrite-free alloy anode enables high-performance Li–S batteries. Adv Sci. 2022. https://doi.org/10.1002/advs.202202204.
Zhou C, Li M, Hu N, et al. Single-atom-regulated heterostructure of binary nanosheets to enable dendrite-free and kinetics-enhanced Li–S batteries. Adv Funct Mater. 2022. https://doi.org/10.1002/adfm.202204635.
Yan M, Wang CY, Fan M, et al. In situ derived mixed Ion/Electron conducting layer on top of a functional separator for high-performance, dendrite-free rechargeable lithium-metal batteries. Adv Funct Mater. 2024. https://doi.org/10.1002/adfm.202301638.
Song J, Jiang Y, Lu Y, et al. A forceful, “Dendrite-Killer” of polyoxomolybdate with reusability effectively dominating dendrite-free lithium metal anode. Small. 2023. https://doi.org/10.1002/smll.202301740.
Senthil C, Kim SG, Kim SS, Hahm MG, Jung HY. Robust, ultrasmooth fluorinated lithium metal interphase feasible via lithiophilic graphene quantum dots for dendrite-less batteries. Small. 2022. https://doi.org/10.1002/smll.202200919.
Liu Z, Dong X, Wen J, Hu P, Shang C. The inducement and “Rejuvenation” of Li dendrites by space confinement and positive Fe/Co-sites. Small. 2023. https://doi.org/10.1002/smll.202300106.
Jeong YC, Kim JH, Nam S, Park CR, Yang SJ. Rational design of nanostructured functional interlayer/separator for advanced Li–S batteries. Adv Funct Mater. 2018. https://doi.org/10.1002/adfm.201707411.
Su Y-S, Manthiram A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat Commun. 2012. https://doi.org/10.1038/ncomms2163.
Ma G, Wen Z, Wang Q, et al. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J Power Sour. 2015. https://doi.org/10.1016/j.jpowsour.2014.09.141.
Fan L, Li M, Li X, **ao W, Chen Z, Lu J. Interlayer material selection for lithium-sulfur batteries. Joule. 2019. https://doi.org/10.1016/j.joule.2019.01.003.
Jiang N, Jiang G, Niu D, et al. Decorating ketjen black with ultra-small Mo2C nanoparticles to enhance polysulfides chemisorption and redox kinetics for lithium-sulfur batteries. J Energy Chem. 2020. https://doi.org/10.1016/j.jechem.2020.04.008.
Hu N, Lv X, Dai Y, Fan L, **ong D, Li X. SnO2/reduced graphene oxide interlayer mitigating the shuttle effect of Li–S batteries. ACS Appl Mater Interfaces. 2018. https://doi.org/10.1021/acsami.8b03255.
Huang Y, Lv D, Zhang Z, et al. Co-Fe bimetallic sulfide with robust chemical adsorption and catalytic activity for polysulfides in lithium-sulfur batteries. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2020.124122.
Guo Y, Li J, Pitcheri R, Zhu J, Wen P, Qiu Y. Electrospun Ti4O7/C conductive nanofibers as interlayer for lithium-sulfur batteries with ultra long cycle life and high-rate capability. Chem Eng J. 2019. https://doi.org/10.1016/j.cej.2018.08.143.
Mo Y, Lin J, Li S, Yu J. Anchoring Mo2C nanoparticles on vertical graphene nanosheets as a highly efficient catalytic interlayer for Li-S batteries. Chem Eng J. 2022. https://doi.org/10.1016/j.cej.2021.134306.
Zhao J, Zhao Y, Yue W-C, et al. V2O3/VN catalysts decorated free-standing multifunctional interlayer for high-performance Li-S battery. Chem Eng J. 2022. https://doi.org/10.1016/j.cej.2022.136082.
Ahn H, Kim Y, Bae J, Kim YK, Kim WB. A multifunctional SnO2-nanowires/carbon composite interlayer for high-performance lithium-sulfur batteries. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2020.126042.
Cheng XB, Hou TZ, Zhang R, et al. Lithium batteries: dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv Mater. 2016. https://doi.org/10.1002/adma.201506124.
Deng N, Liu Y, Li Q, et al. Functional mechanism analysis and customized structure design of interlayers for high performance Li-S battery. Energy Storage Mater. 2019. https://doi.org/10.1016/j.ensm.2019.04.042.
Li Q, Zeng F-L, Guan Y-P, et al. Poly (dimethylsiloxane) modified lithium anode for enhanced performance of lithium-sulfur batteries. Energy Storage Mater. 2018. https://doi.org/10.1016/j.ensm.2018.01.002.
**e K, Yuan K, Zhang K, et al. Dual functionalities of carbon nanotube films for dendrite-free and high energy–high power lithium–sulfur batteries. ACS Appl Mater Interfaces. 2017. https://doi.org/10.1021/acsami.6b14039.
Zhao Y, Sun Q, Li X, et al. Carbon paper interlayers: a universal and effective approach for highly stable Li metal anodes. Nano Energy. 2018. https://doi.org/10.1016/j.nanoen.2017.11.032.
He Y, Zhao Y, Zhang Y, et al. Building flexibly porous conductive skeleton inlaid with surface oxygen-dominated MXene as an amphiphilic nanoreactor for stable Li-S pouch batteries. Energy Storage Mater. 2022. https://doi.org/10.1016/j.ensm.2022.02.006.
Etxebarria A, Koch SL, Bondarchuk O, Passerini S, Teobaldi G, Muñoz-Márquez MÁ. Work function evolution in Li anode processing. Adv Energy Mater. 2020. https://doi.org/10.1002/aenm.202000520.
** Y, Zhou G, Shi F, et al. Reactivation of dead sulfide species in lithium polysulfide flow battery for grid scale energy storage. Nat Commun. 2017. https://doi.org/10.1038/s41467-017-00537-0.
Song B, Su L, Liu X, et al. An examination and prospect of stabilizing Li metal anode in lithium–sulfur batteries: a review of latest progress. Electron. 2023. https://doi.org/10.1002/elt2.13.
Liu Z, Chen M, Zhou D, **ao Z. Scavenging of “Dead Sulfur” and “Dead Lithium” revealed by integrated-heterogeneous catalysis for advanced lithium-sulfur batteries. Adv Funct Mater. 2023. https://doi.org/10.1002/adfm.202306321.
Acknowledgements
This work was supported by R&D Program for Forest Science Technology (Project No. 2023499B10-2325-AA02) provided by the Korea Forest Service (Korea Forestry Promotion Institute), Semiconductor-Secondary Battery Interfacing Platform Technology Development Project of NNFC, and National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A5A1033719).
Author information
Authors and Affiliations
Contributions
S.H., J.H.L. and J.K. contributed equally to this work. S.H. and J.H.L. wrote the main manuscript text. J.K. conceptualized and revised the manuscript. J.L. reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, S., Lee, J.H., Kim, J. et al. Recent advances in li metal anode protection for high performance lithium-sulfur batteries. Discov Chem Eng 4, 9 (2024). https://doi.org/10.1007/s43938-024-00045-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00045-w