Abstract
Background
Multiple sclerosis (MS) is a devastating autoimmune disorder characterized by oligodendrocytes (OLGs) loss and demyelination. In this study, we have examined the effects of metformin (MET) on the oligodendrogenesis, redox signaling, apoptosis, and glial responses during a self-repairing period (1-week) in the animal model of MS.
Methods
For induction of demyelination, C57BL/6 J mice were fed a 0.2% cuprizone (CPZ) for 5 weeks. Thereafter, CPZ was removed for 1-week and molecular and behavioral changes were monitored in the presence or absence of MET (50 mg/kg body weight/day).
Results
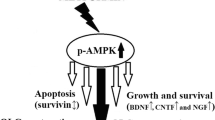
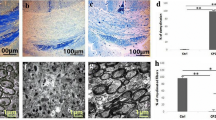
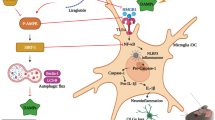
MET remarkably increased the localization of precursor OLGs (NG2+/O4+ cells) and subsequently the renewal of mature OLGs (MOG+ cells) in the corpus callosum via AMPK/mammalian target of rapamycin (mTOR) pathway. Moreover, we observed a significant elevation in the antioxidant responses, especially in mature OLGs (MOG+/nuclear factor erythroid 2-related factor 2 (Nrf2+) cells) after MET intervention. MET also reduced brain apoptosis markers and lessened motor dysfunction in the open-field test. While MET was unable to decrease active astrogliosis (GFAP mRNA), it reduced microgliosis by down-regulation of Mac-3 mRNA a marker of pro-inflammatory microglia/macrophages. Molecular modeling studies, likewise, confirmed that MET exerts its effects via direct interaction with AMPK.
Conclusions
Altogether, our study reveals that MET effectively induces lesion reduction and elevated molecular processes that support myelin recovery via direct activation of AMPK and indirect regulation of AMPK/Nrf2/mTOR pathway in OLGs. These findings facilitate the development of new therapeutic strategies based on AMPK activation for MS in the near future.










Similar content being viewed by others
References
Sanadgol N, Zahedani SS, Sharifzadeh M, Khalseh R, Barbari GR, Abdollahi M. Recent updates in imperative natural compounds for healthy brain and nerve function: a systematic review of implications for multiple sclerosis. Curr Drug Targets. 2017;18(13):1499–517.
Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85.
Annibali V, Mechelli R, Romano S, Buscarinu MC, Fornasiero A, Umeton R, Ricigliano VA, Orzi F, Coccia EM, Salvetti M, Ristori G. IFN-β and multiple sclerosis: from etiology to therapy and back. Cytokine Growth Factor Rev. 2015;26(2):221–8.
Prantner D, Perkins DJ, Vogel SN. AMP-activated kinase (AMPK) promotes innate immunity and antiviral defense through modulation of stimulator of interferon genes (STING) signaling. J Biol Chem. 2017;292(1):292–304.
Meares GP, Qin H, Liu Y, Holdbrooks AT, Benveniste EN. AMP-activated protein kinase restricts IFN-gamma signaling. J Immunol. 2013;190(1):372–80.
Lyons C, Roche H. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int J Mol Sci. 2018;19(10):E3092.
Potter WB, O’Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS ONE. 2010;5(2):e8996.
Evans AM, Mahmoud AD, Moral-Sanz J, Hartmann S. The emerging role of AMPK in the regulation of breathing and oxygen supply. Biochem J. 2016;473(17):2561–72.
Zimmermann K, Baldinger J, Mayerhofer B, Atanasov AG, Dirsch VM, Heiss EH. Activated AMPK boosts the Nrf2/HO-1 signaling axis—a role for the unfolded protein response. Free Radic Biol Med. 2015;88(Pt B):417–26.
Ju TC, Chen HM, Chen YC, Chang CP, Chang C, Chern Y. AMPK-alpha1 functions downstream of oxidative stress to mediate neuronal atrophy in Huntington’s disease. Biochim Biophys Acta. 2014;1842(9):1668–80.
Kim JE, Kim YW, Lee IK, Kim JY, Kang YJ, Park SY. AMP-activated protein kinase activation by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) inhibits palmitate-induced endothelial cell apoptosis through reactive oxygen species suppression. J Pharmacol Sci. 2008;106(3):394–403.
Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27(12):2627–33.
Singh I, Samuvel DJ, Choi S, Saxena N, Singh AK, Won J. Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model. Immunology. 2018;154(3):434–51.
Nath N, Khan M, Rattan R, Mangalam A, Makkar RS, de Meester C, Bertrand L, Singh I, Chen Y, Viollet B, Giri S. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun. 2009;386(1):16–20.
Blazquez C, Geelen MJ, Velasco G, Guzmán M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489(2–3):149–53.
Paintlia AS, Paintlia MK, Mohan S, Singh AK, Singh I. AMP-activated protein kinase signaling protects oligodendrocytes that restore central nervous system functions in an experimental autoimmune encephalomyelitis model. Am J Pathol. 2013;183(2):526–41.
Sanadgol N, Golab F, Askari H, Moradi F, Ajdary M, Mehdizadeh M. Alpha-lipoic acid mitigates toxic-induced demyelination in the corpus callosum by lessening of oxidative stress and stimulation of polydendrocytes proliferation. Metab Brain Dis. 2018;33(1):27–37.
Sanadgol N, Golab F, Mostafaie A, Mehdizadeh M, Abdollahi M, Sharifzadeh M, Ravan H. Ellagic acid ameliorates cuprizone-induced acute CNS inflammation via restriction of microgliosis and down-regulation of CCL2 and CCL3 pro-inflammatory chemokines. Cell Mol Biol (Noisy-le-grand). 2016;62(12):24–30.
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171(13):3146–57.
Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–52.
Sanadgol N, Golab F, Mostafaie A, Mehdizadeh M, Khalseh R, Mahmoudi M, Abdollahi M, Vakilzadeh G, Taghizadeh G, Sharifzadeh M. Low, but not high, dose triptolide controls neuroinflammation and improves behavioral deficits in toxic model of multiple sclerosis by dampening of NF-κB activation and acceleration of intrinsic myelin repair. Toxicol Appl Pharmacol. 2018;342:86–98.
Vakilzadeh G, Khodagholi F, Ghadiri T, Darvishi M, Ghaemi A, Noorbakhsh F, Gorji A, Sharifzadeh M. Protective effect of a cAMP analogue on behavioral deficits and neuropathological changes in cuprizone model of demyelination. Mol Neurobiol. 2015;52(1):130–41.
Ramroodi N, Khani M, Ganjali Z, Javan MR, Sanadgol N, Khalseh R, Ravan H, Sanadgol E, Abdollahi M. Prophylactic effect of BIO-1211 small-molecule antagonist of VLA-4 in the EAE mouse model of multiple sclerosis. Immunol Invest. 2015;44(7):694–712.
Sanadgol N, Golab F, Tashakkor Z, Taki N, Moradi Kouchi S, Mostafaie A, Mehdizadeh M, Abdollahi M, Taghizadeh G, Sharifzadeh M. Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharm Biol. 2017;55(1):1679–87.
Baeeri M, Momtaz S, Navaei-Nigjeh M, Niaz K, Rahimifard M, Ghasemi-Niri SF, Sanadgol N, Hodjat M, Sharifzadeh M, Abdollahi M. Molecular evidence on the protective effect of ellagic acid on phosalone-induced senescence in rat embryonic fibroblast cells. Food Chem Toxicol. 2017;100:8–23.
Askari H, Seifi B, Kadkhodaee M, Sanadgol N, Elshiekh M, Ranjbaran M, Ahghari P. Protective effects of hydrogen sulfide on chronic kidney disease by reducing oxidative stress, inflammation and apoptosis. EXCLI J. 2018;17:14–23.
Askari H, Abazari MF, Ghoraeian P, Torabinejad S, Nouri Aleagha M, Mirfallah Nassiri R, Tahmasebi F, Abedi N, Rajani SF, Salarian A, Belaran M, Elshiekh M, Sanadgol N. Ameliorative effects of hydrogen sulfide (NaHS) on chronic kidney disease-induced brain dysfunction in rats: implication on role of nitric oxide (NO) signaling. Metab Brain Dis. 2018;33(6):1945–54.
Aghajani M, Vaez Mahdavi MR, Najafabadi MK, Ghazanfari T, Moradi F, Golchoobian R, Askari H, Sanadgol N, Moghaddam EK. Depressed immune responses and accelerated splenic apoptosis due to experience of food deprivation and inequality but not unstable social status in Balb/c mice. NeuroImmunoModulation. 2017;24(4–5):200–10.
Shirazi MK, Azarnezhad A, Abazari MF, Poorebrahim M, Ghoraeian P, Sanadgol N, Bokharaie H, Heydari S, Abbasi A, Kabiri S, Aleagha MN, Enderami SE, Dashtaki AS, Askari H. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: involvement of oxidative stress, autophagy and apoptosis. J Cell Physiol. 2019;234(7):11411–23.
Hosseini A, Sharifzadeh M, Rezayat SM, Hassanzadeh G, Hassani S, Baeeri M, Shetab-Bushehri V, Kuznetsov DA, Abdollahi M. Benefit of magnesium-25 carrying porphyrin-fullerene nanoparticles in experimental diabetic neuropathy. Int J Nanomed. 2010;5:517–23.
Poorebrahim M, Asghari M, Abazari MF, Askari H, Sadeghi S, Taheri-Kafrani A, Nasr-Esfahani MH, Ghoraeian P, Aleagha MN, Arab SS, Kennedy D, Montaseri A, Mehranfar M, Sanadgol N. immunomodulatory effects of a rationally designed peptide mimetic of human Ifnβ in Eae model of multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:49–61.
Robinson AP, Rodgers JM, Goings GE, Miller SD. Characterization of oligodendroglial populations in mouse demyelinating disease using flow cytometry: clues for MS pathogenesis. PLoS ONE. 2014;9(9):e107649.
Jennings AR, Carroll WM. Oligodendrocyte lineage cells in chronic demyelination of multiple sclerosis optic nerve. Brain Pathol. 2015;25(5):517–30.
Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev. 2014;47:485–505.
Agarwal S, Agarwal S, Tiwari SK, Seth B, Yadav A, Singh A, Mudawal A, Chauhan LK, Gupta SK, Choubey V, Tripathi A, Kumar A, Ray RS, Shukla S, Parmar D, Chaturvedi RK. Activation of autophagic flux against xenoestrogen bisphenol-A-induced hippocampal neurodegeneration via AMP kinase (AMPK)/mammalian target of rapamycin (mTOR) pathways. J Biol Chem. 2015;290(34):21163–84.
Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23–35.
Caprariello AV, Mangla S, Miller RH, Selkirk SM. Apoptosis of oligodendrocytes in the central nervous system results in rapid focal demyelination. Ann Neurol. 2012;72(3):395–405.
Gong X, Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X, Lu G, Zhang S. miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am J Transl Res. 2016;8(5):2127–37.
Peixoto CA, Oliveira WH, Araújo SMDR, Nunes AKS. AMPK activation: role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 2017;298(Pt A):31–41.
Xue H, Xue H, Ji Y, Wei S, Yu Y, Yan X, Liu S, Zhang M, Yao F, Lan X, Chen L. HGSD attenuates neuronal apoptosis through enhancing neuronal autophagy in the brain of diabetic mice: the role of AMP-activated protein kinase. Life Sci. 2016;153:23–34.
Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the develo** rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17(1):45–58.
Zhou Z, Chen S, Zhao H, Wang C, Gao K, Guo Y, Shen Z, Wang Y, Wang H, Mei X. Probucol inhibits neural cell apoptosis via inhibition of mTOR signaling pathway after spinal cord injury. Neuroscience. 2016;329:193–200.
Zheng X, Boyer L, ** M, Kim Y, Fan W, Bardy C, Berggren T, Evans RM, Gage FH, Hunter T. Alleviation of neuronal energy deficiency by mTOR inhibition as a treatment for mitochondria-related neurodegeneration. eLife. 2016;5:e13378.
Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(Pt 7):1914–24.
Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135(Pt 3):886–99.
French HM, French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87(14):3076–87.
Wang S, Zhang M, Liang B, Xu J, **e Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106(6):1117–28.
Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH, He ZX, Zhou ZW, **ao G, Yang YX, Zhang X, Yang T, Chen XW, Qiu JX, Zhou SF. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Dev Ther. 2015;9:1555–84.
Zhang H, Liu YY, Jiang Q, Li KR, Zhao YX, Cao C, Yao J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic Biol Med. 2014;69:219–28.
Huang H, Taraboletti A, Shriver LP. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol. 2015;5:169–75.
Jia Y, Jia Y, Wang H, Wang Q, Ding H, Wu H, Pan H. Silencing Nrf2 impairs glioma cell proliferation via AMPK-activated mTOR inhibition. Biochem Biophys Res Commun. 2016;469(3):665–71.
van Horssen J, Drexhage JA, Flor T, Gerritsen W, van der Valk P, de Vries HE. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic Biol Med. 2010;49(8):1283–9.
Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, **ao H. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20(4):574–88.
Shibata T, Saito S, Kokubu A, Suzuki T, Yamamoto M, Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70(22):9095–105.
Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747–54.
Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adultcentral nervous system. J Anat. 2005;207(6):707–16.
El Waly B, Macchi M, Cayre M, Durbec P. Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci. 2014;8:145.
Kempf A, Schwab ME. Nogo-A represses anatomical and synaptic plasticity in the central nervous system. Physiology. 2013;28(3):151–63.
Yang Y, Liu Y, Wei P, Peng H, Winger R, Hussain RZ, Ben LH, Cravens PD, Gocke AR, Puttaparthi K, Racke MK, McTigue DM, Lovett-Racke AE. Silencing Nogo-A promotes functional recovery in demyelinating disease. Ann Neurol. 2010;67(4):498–507.
Houshmand F, Sanadgol N, Barati M, Golab F, Ramezani-sefidar S, Tanbakooie S, Tabatabaei M, Amiri M. Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2 + precursor cells in the cuprizone murine model of multiple sclerosis. DARU J Pharm Sci. 2019. https://doi.org/10.1007/s40199-019-00286-z.
Largani SHH, Borhani-Haghighi M, Pasbakhsh P, Mahabadi VP, Nekoonam S, Shiri E, Kashani IR, Zendehdel A. Oligoprotective effect of metformin through the AMPK-dependent on restoration of mitochondrial hemostasis in the cuprizone-induced multiple sclerosis model. J Mol Histol. 2019;50(3):263–71.
Acknowledgements
We are grateful to Dr. M. Baeiri from the Institute of Pharmaceutical Sciences (TIPS) at TUMS for her technical support.
Funding
This work was supported by the Iran University of Medical Sciences and University of Zabol (UOZ-GR-9618-5).
Author information
Authors and Affiliations
Contributions
NS, FG, and FH, conceived and designed the study. All authors contributed to performing the relevant experiments. NS, MB, FG, and FH, analyzed the data. NS, and FG, wrote the paper. All authors contributed to reviewing and editing the manuscript and also read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no potential conflict of interest or competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Sanadgol, N., Barati, M., Houshmand, F. et al. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol. Rep 72, 641–658 (2020). https://doi.org/10.1007/s43440-019-00019-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-019-00019-8