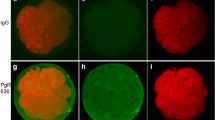
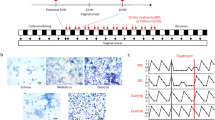
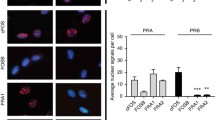
Abstract
Classic transcriptional regulation by progesterone via the nuclear progesterone receptors A and B (PR-A, PR-B) has been recognized for decades. Less attention has been given to a mitochondrial progesterone receptor (PR-M) responsible for non-nuclear activities. PR-M is derived from the progesterone receptor (PR) gene from an alternate promoter with the cDNA encoding a unique 5′ membrane binding domain followed by the same hinge and hormone-binding domain of the nPR. The protein binds to the mitochondrial outer membrane and functions to increase cellular respiration via increased beta-oxidation and oxidative phosphorylation with resulting adenosine triphosphate (ATP) production. Physiologic activities of PR-M have been studied in cardiac function, spermatozoa activation, and myometrial growth, all known to respond to progesterone. Progesterone via PR-M increases cardiomyocyte cellular respiration to meet the metabolic demands of pregnancy with increased contractility. Consequential gene changes associated with PR-M activation include production of proteins for sarcomere development and for fatty acid oxidation. Regarding spermatozoa function, progesterone via PR-M increases cellular energy production necessary for progesterone-dependent hyperactivation. A role of progesterone in myometrial and leiomyomata growth may also be explained by the increase in necessary cellular energy for proliferation. Lastly, the multi-organ increase in cellular respiration may contribute to the progesterone-dependent increase in metabolic rate reflected by an increase in body temperature through compensatory non-shivering thermogenesis. An evolutionary comparison shows PR-M expressed in humans, apes, and Old World monkeys, but the necessary gene sequence is absent in New World monkeys and lower species. The evolutionary advantage to PR-M remains to be defined, but its presence may enhance catabolism to support the extended gestation and brain development found in these primates.






Similar content being viewed by others
References
Allen W, Corner G. Physiology of the corpus luteum, III: normal growth and implantation of embryos after very early ablation of the ovaries, and under the influence of extracts of the corpus luteum. Am J Physiol. 1929;88:340–6.
Ali S, Balachandran K, O’Malley B. 90 years of progesterone: ninety years of progesterone: the ‘other’ ovarian hormone. J Mol Endocrinol. 2020;65:E1–4.
Conneely O, Sullivan W, Toft D, Birnbaumer M, Cook R, Maxwell B, et al. Molecular cloning of the chicken progesterone receptor. Science. 1986;233:767–70.
Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zoology. 1971;177:129–45.
Improta-Bears T, Whorton A, Codazzi F, York J, Meyer T, McDonnell D. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci U S A 1999;96:4686-91.
Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–80.
Saner K, Welter B, Zhang F, Hansen E, Dupont B, Wei Y, et al. Cloning and expression of a novel, truncated progesterone receptor. Mol Cell Endocrinol. 2003;200:155–63.
Price TM, Dai Q. The role of a mitochondrial progesterone receptor (PR-M) in progesterone action. Sem Reprod Med. 2015;33:185–94.
Misrahi M, Atger M, d’Auriol L, Loosfelt H, Meriel C, Fridlansky F, et al. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophysical Res Com. 1987;143:740–8.
Wen D, Xu Y, Mais D, et al. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–64.
Onate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai M-J, Edwards DP, et al. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors*. J Biol Chem. 1998;273:12101–8.
Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different progesterone receptor forms A and B. EMBO. 1990;9:1603–14.
Sartorius C, Shen T, Horwitz KB. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Ca Res Treat. 2003;79:287–99.
Kaluka D, Batabyal D, Chiang B-Y, Poulos TL, Yeh S-R. Spectroscopic and mutagenesis studies of human PGRMC1. Biochemistry. 2015;54:1638–47.
Cahill MA, Medlock AE. Thoughts on interactions between PGRMC1 and diverse attested and potental hydrophobic ligands. J Steroid Biochem Mol Biol. 2017;171:11–33.
Oda S, Nakajima M, Toyoda Y, Fukami T, Yokoi T. Progesterone receptor membrane component 1 modulates human cytochrome P450 activities in an isoform-dependent manner. Drug Metab Dispo. 2011;39:2057–65.
Peluso JJ, Pru CA, Liu X, Kelp NC, Pru JK. Progesterone receptor membrane component 1 and 2 regulate granulosa cell mitosis and survival through a NFΚB-dependent mechanism†. Biol Reprod. 2019;100:1571–80.
Guo M, Zhang C, Wang Y, Feng L, Wang Z, Niu W, et al. Progesterone receptor membrane component 1 mediates progesterone-induced suppression of oocyte meiotic prophase i and primordial folliculogenesis. Sci Rep. 2016;6:36869.
Zhu Y, Rice C, Pang Y, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci. 2003;100:2231–6.
Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL. The role of xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol. 2007;21:664–73.
Dai Q, Shah AA, Garde RV, Yonish BA, Zhang L, Medvitz NA, et al. A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol Endocrinol. 2013;27:741–53.
Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009;297:E1089–96.
Ouzounian J, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30:317–29.
Dai Q, Likes C, Luz A, Mao L, Yeh J, Wei Z, et al. A mitochondrial progesterone receptor increase cardia beta-oxidation and remodeling. J Endocrine Soc. 2019;3:446–67.
Morano I, Hadicke K, Haase H, Bohm M, Erdmann E, Schaub M. Changes in essential myosin light chain isoform expression provide a molecular basis for isometric force regulation in the failing human heart. J Mol Cell Cardiol. 1997;29:1177–87.
Lebenstedt M, Petra P, Karl-Martin P. Reduced metabolic rate in athletes with menstrual disorders. Med Sci Sports Exerc. 1999;31:1250–6.
Gavrilova-Jordan L, Price T. Actions of steroids in mitochondria. Sem Reprod Med. 2007;25:154-164
Kobayashi A, Azuma K, Ikeda K, Inoue S. Mechanisms underlying the regulation of mitochondrial respiratory chain complexes by nuclear steroid receptors. Int J Mol 2020;21(18):6683
Davis PJ, Leonard JL, Lin HY, Leinung M, Mousa SA. Molecular basis of nongenomic actions of thyroid hormone. Vitam Horm . 2018;106:67-96.
Bisdee J, James W, Shaw M. Changes in energy expenditure during the menstrual cycle. Br J Nutr. 1989;61:187–99.
Meijer GA, Westerterp KR, Saris WH, ten Hoor F. Slee** metabolic rate in relation to body composition and the menstrual cycle. Am J Clin Nutri. 1992;55:637–40.
Koop-Hoolihan L, van Loan D, Wong W, King J. Longitudinal assessment of energy balance in well-nourished, pregnant women. Am J Clin Nutri. 1999;69:697–704.
Gupta RD, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: from bench to bedside. Indian J Endocrinol Metabol. 2017;21:594–9.
Steward RG, Bateman LA, Slentz C, Stanczyk FZ, Price TM. The impact of short-term depot-medroxyprogesterone acetate treatment on resting metabolic rate. Contraception. 2016;93:317–22.
Stephenson L, Kolka M. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol. 1985;249:R186–91.
Tsai C, Matsumura K, Nakayama T. Effects of progesterone on thermosensitive neurons in preoptic slice preparations. Neurosci Lett. 1988;86:56–60.
Monjo M, Rodríguez AM, Palou A, Roca P. Direct effects of testosterone, 17β-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinol. 2003;144:4923–30.
Blondin DP, Haman F. Chapter 10 - shivering and nonshivering thermogenesis in skeletal muscles. Handbook of clinical neurology. A. A. Romanovsky, Elsevier; 2018; 156:153–173.
Rodríguez AM, Monjo M, Roca P, Palou A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol Life Sci CMLS. 2002;59:1714–23.
Smith W, Broadbridge R, East J, Lee A. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem J. 2002;361:277–86.
Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–9.
Teves ME, Barbano F, Guidobaldi HA, Sanchez R, Miska W, Giojalas LC. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil Steril. 2006;86:745–9.
Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol Cell Endocrinol. 2009;308:39–46.
Contreras HR, Llanos MN. Detection of progesterone receptors in human spermatozoa and their correlation with morphological and functional properties. Int J Androl. 2001;24:246–52.
Tamburrino L, Marchiani S, Muratori M, Luconi M, Baldi E. Progesterone, spermatozoa and reproduction: an updated review. Mol Cell Endocrinol. 2020;516:110952.
Williams M, Hill CJ, Scudamore I, Dunphy B, Cooke ID, Barratt CLR. Physiology: sperm numbers and distribution within the human Fallopian tube around ovulation. Hum Reprod. 1993;8:2019–26.
Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, et al. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science. 2016;352:555–9.
**a J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation1. Biol Reprod. 2007;77:551–9.
Tantibhedhyangkul J, Hawkins KC, Dai Q, Mu K, Dunn CN, Miller SE, et al. Expression of a mitochondrial progesterone receptor in human spermatozoa correlates with a progestin-dependent increase in mitochondrial membrane potential. Androl. 2014;2:875–83.
Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev. 2017;4(4):CD010770. https://doi.org/10.1002/14651858.CD010770.pub2
Bouchard P, Chabbert-Buffet N, Fauser BCJM. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fert Steril. 2011;96:1175–89.
Carr B, Marshburn P, Weatherall P, Bradshaw K, Breslau N, Byrd W, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo- controlled, crossover trial. J Clin Endocrinol Metab. 1993;76:1217–23.
Stanfield Z, Amini P, Wang J, Yi L, Tan H, Chance MR, et al. Interplay of transcriptional signaling by progesterone, cyclic AMP, and inflammation in myometrial cells: implications for the control of human parturition. Mol Hum Reprod. 2019;25:408–22.
Markowski DN, Bartnitzke S, Löning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids—their relationship to cytogenetic subgroups. Eur J Hum Genet. 2012;131:1528–36.
Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, et al. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Pathol. 2014;27:1144–53.
Tomlinson IPM, Alam NA, Rowan AJ, Barclay E, Jaeger EEM, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10.
Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci. 2007;104:18700–5.
Wang R, Sheehan PM, Brennecke SP. Changes in myometrial expression of progesterone receptor membrane components 1 and 2 are associated with human parturition at term Reprod. Fert Develop. 2016;28:618–27.
Kowalik MK, Dobrzyn K, Rekawiecki R, Kotwica J. Expression of membrane progestin receptors (mPRs) α, β and γ in the bovine uterus during the oestrous cycle and pregnancy. Theriogenology. 2019;140:171–9.
Feng Q, Crochet JR, Dai Q, Leppert PC, Price TM. Expression of a mitochondrial progesterone receptor (PR-M) in leiomyomata and association with increased mitochondrial membrane potential. J Clin Endocrinol Metabol. 2014;99:E390–9.
Ferenczy A, Richart RM, Okagaki T. A comparative ultrastructural study of leiomyosarcoma, cellular leiomyoma, and leiomyoma of the uterus. Cancer. 1971;28:1004–18.
Sahebkar A, Simental-Mendía LE, Pedone C, Ferretti G, Nachtigal P, Bo S, et al. Statin therapy and plasma free fatty acids: a systematic review and meta-analysis of controlled clinical trials. Br J Clin Pharmacol. 2016;81:807–18.
Borahay MA, Fang X, Baillargeon JG, Kilic GS, Boehning DF, Kuo Y-F. Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study. Am J Obstet Gynecol. 2016;215:750.e1-.e8.
Shen Z, Li S, Sheng B, Shen Q, Sun L-Z, Zhu H, et al. The role of atorvastatin in suppressing tumor growth of uterine fibroids. J Transl Med. 2018;16:53.
Thornton J. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Nat Acad Sci. 2001;98:5671–6.
Martin R. Reproductive characteristics of New World monkeys. Int Zoo Yb. 2012;46:95–108.
Barton R, Capellini I. Maternal investment, life histories, and the costs of brain growth in mammals. Proc Nat Acad Sci. 2011;108:6169–74.
Pontzer H, Brown MH, Raichlen DA, Dunsworth H, Hare B, Walker K, et al. Metabolic acceleration and the evolution of human brain size and life history. Nature. 2016;533:390–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shaia, K.L., Harris, B.S., Selter, J.H. et al. Reproductive Functions of the Mitochondrial Progesterone Receptor (PR-M). Reprod. Sci. 30, 1443–1452 (2023). https://doi.org/10.1007/s43032-022-01092-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01092-w