Abstract
Cas12a (Cpf1), a Class 2 Type V CRISPR/Cas nuclease, has several unique attributes for genome editing and may provide a valuable alternative to Cas9. However, a low editing efficiency due to temperature sensitivity and insufficient cleavage activity of the Cas12a nuclease are major obstacles to its broad application. In this report, we generated two variants, ttAsCas12 Ultra and ttLbCas12a Ultra harboring three (E174R, M537R, and F870L) or two (D156R and E795L) mutations, respectively, by combining the mutations from the temperature-tolerant variants ttAsCas12a (E174R) and ttLbCas12a (D156R), and those from the highly active variants AsCas12a Ultra (M537R and F870L) and LbCas12a Ultra (E795L). We compared editing efficiencies of the five resulting Cas12a variants (LbCas12a, ttLbCas12a, ttLbCas12a Ultra, AsCas12a Ultra, and ttAsCas12 Ultra) at six target sites of four genes in Arabidopsis (Arabidopsis thaliana). The variant ttLbCas12a Ultra, harboring the D156R and E795L mutations, exhibited the highest editing efficiency of all variants tested in Arabidopsis and can be used to generate homozygous or biallelic mutants in a single generation in Arabidopsis plants grown at 22 °C. In addition, optimization of ttLbCas12a Ultra, by varying nuclear localization signal sequences and codon usage, further greatly improved editing efficiency. Collectively, our results indicate that ttLbCas12a Ultra is a valuable alternative to Cas9 for editing genes or promoters in Arabidopsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cas12a (Cpf1), an endonuclease from Class 2 Type V clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease (Cas) systems, has several unique attributes as a genome-editing tool (Makarova et al. 2020; Zetsche et al. 2015). First, unlike Class 2 Type II CRISPR/Cas systems, Cas12a nucleases are complexed with a single CRISPR RNA (crRNA) that does not require a trans-activating CRISPR RNA (tracrRNA) (Zetsche et al. 2015). The crRNA of Cas12a (∼42 nt) is shorter than the single guide RNA (sgRNA) of Cas9 (∼100 nt), making it more amenable to chemical synthesis and easier to deliver with other CRISPR reagents into cells as ribonucleoprotein (RNP) complexes (Banakar et al. 2020; Su et al. 2023). Second, identified Cas12a nucleases, such as LbCas12a from Lachnospiraceae bacterium and AsCas12a from Acidaminococcus sp., typically recognize a T-rich protospacer adjacent motif (PAM) sequence at the 5' end of the protospacer (Zetsche et al. 2015). This attribute facilitates targeting AT-rich genomic regions that would be challenging to target with the most commonly used SpCas9 from Streptococcus pyogenes, which prefers G-rich PAMs (Zetsche et al. 2015). Third, Cas12a induces double-strand breaks (DSBs) with 5' overhangs that are distal from its PAM sequence (Zetsche et al. 2015). Since Cas12a-induced mutations are distal from the PAM, and mismatches distal from the PAM between the guide sequence of the crRNA and its target site in genomic DNA are more frequently tolerated by Cas9 or Cas12, Cas12a may potentially repeatedly induce DSBs even after the initial target site has been altered via imprecise DNA repair. Consequently, mutations induced by Cas12a are biased toward larger deletions compared to the mutations typically produced by Cas9 (Zetsche et al. 2015). In addition, repeated DNA cleavage facilitates releasing homology-directed repair (HDR) donors from genomic DNA and prolonging the time-window of HDR prior to repair via the non-homologous end joining (NHEJ) pathway. Finally, Cas12a proteins can process precursor crRNA (pre-crRNA) arrays, which can be harnessed for multiplex genome editing from a single transcript (Zetsche et al. 2017). Thus, Cas12a-induced genome editing could be a valuable alternative to Cas9.
Like Cas9, Cas12a has been employed for genome editing in plants (Bernabe-Orts et al. 2019; Lee et al. 2019; Li et al. 2018, 2021), and the ttLbCas12a Ultra sequence was codon-optimized with monocot plants, we generated another version of ttLbCas12a Ultra that harbored the same NLS sequences as in PEmax (Chen et al. 2021) and was codon-optimized for dicot species, which we named ttLbCas12a Ultra V2 (Fig. 3C; Table S6). The optimized NLS sequences were composed of a bipartite SV40 NLS at both terminals and an additional c-Myc NLS at C-terminal. Indeed, we obtained much more edited plants when expressing ttLbCas12a Ultra V2 than with ttLbCas12a Ultra, indicating that editing efficiency can be further improved in Arabidopsis by optimizing NLS sequences and the codon usage of the nuclease gene (Fig. 3D; Table S6).
Mutations in T1 plants are heritable to the subsequent generation
The mCherry cassette in the Cas12a vectors provided an effective strategy for reliably isolating Cas12-free Arabidopsis plants (Gao et al. 2016). Indeed, seeds harboring the transgene with the mCherry cassette display strong red fluorescence, while the absence of red fluorescence indicates segregation of the T-DNA, resulting in T-DNA-free seeds (Gao et al. 2016). Before analyzing the heritability of each mutation, we first isolated T-DNA-free seeds, based on the lack of red fluorescence.
To test whether mutations at the ECA3-1 and ECA3-2 target sites are heritable, we isolated T-DNA-free T2 seeds from six and nine T1 biallelic mutant plants, respectively. Since Cas12a-induced mutations at the ECA3-1 and ECA3-2 target sites disrupted the BglII and EcoRV restriction enzyme sites, respectively, we employed restriction fragment length polymorphism (RFLP) analysis to identify mutations. RFLP analysis indicated that all T-DNA-free T2 plants harbor homozygous or biallelic mutations, as evidenced by the lack of DNA cleavage (Fig. S1, S2; Table S7). These results demonstrate that mutations in ECA3 present in the T1 plants are heritable.
To provide evidence that mutations at the GL2 target are heritable, we isolated 30 T-DNA-free T2 seeds from the only identified T1 biallelic mutant plant, which surprisingly did not exhibit a glabrous phenotype (Table S7). High-throughput sequencing analysis of the resulting 30 T2 plants showed that they carry homozygous or biallelic mutations identical to the two mutations detected in the T1 plant (Table S8). We observed that six plants homozygous for an 8-bp deletion at the target site exhibit the glabrous phenotype (Fig. 4A), whereas the remaining six plants homozygous for a 21-bp insertion and 18 plants with biallelic mutations did not show a glabrous phenotype (Table S8). These results indicate that the 21-bp insertion, which leads to an insertion of seven amino acids, does not affect GL2 function, explaining the lack of a glabrous phenotype of the original T1 plant. These results do, however, demonstrate that the introduced mutations in GL2 are heritable.
Phenotypes of T-DNA-free homozygous gl2 or tt4 mutants derived from T1 plants harboring ttLbCas12a Ultra and the U6-tRNA cassette. A Glabrous phenotype of a T-DNA-free T2 homozygous gl2 mutant (right) compared to the wild type (WT; left). B Yellow seed coat phenotype of a T-DNA-free T3 homozygous tt4 mutant (right) compared to the WT (left)
We performed a similar analysis of mutations at the TT4 target site; to this end, we isolated T-DNA-free T2 seeds from seven T1 homozygous or biallelic mutant plants. All T2 seeds were yellow rather than dark brown (Fig. 4B and Table S8), a phenotype typical of tt4 mutants (Malzahn et al. 2019). We conclude that all T2 progeny are homozygous or biallelic tt4 mutants, demonstrating that mutations present in T1 plants at the TT4 target site are heritable.
We also isolated T-DNA-free T2 seeds from ten (GL1-1) and five (GL1-2) T1 phenotypically homozygous or biallelic mutant plants in which GL1 was targeted for editing (Table S10). We determined that all T2 plants are completely glabrous (Table S10). Sanger sequencing of the target site in individual T2 plants confirmed that they harbor homozygous or biallelic mutations in GL1 (Tables S11, S12). These results demonstrate that mutations present in T1 plants at the GL1 locus are heritable.
Off-target mutations were not detected in mutant plants
High editing efficiency usually means high off-target mutagenesis. We selected the most efficient two targets, GL1-1 and GL1-2, to analyze off-target mutations. We searched potential off-target sites of the GL1-1 target and obtained two harboring 3 or 4 mismatches in At5G40330 or At1G22640, respectively (Table S13). We also obtained an off-target site with 5 mismatches of the GL1-2 target (Table S13). We amplified PCR fragments spanning off-target sites from 36 gl1-1 and 36 gl1-2 T1 mutant plants that harbor homozygous or biallelic mutations and analyzed mutations by Sanger sequencing. We detected no off-target mutations (Table S13), indicating that careful selection of targets will be able to avoid off-target mutagenesis induced by highly efficient LbCas12a variants.
Discussion
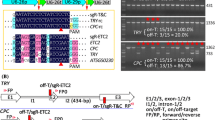
Temperature sensitivity and insufficient cleavage activity of Cas12a nucleases limit editing efficiency of targets in plants and many efforts have been made to improve these restrictions (Guo et al. 2022; Huang et al. 2022; Lee et al. 2019; Liu et al. 2019a; Ma et al. 2022; Zhang et al. 2023, 2021). Two LbCas12a Ultra variants have previously been reported: one harbors a single E795L mutation (Zhang et al. 2021) and another harbors the two mutations N527R and E795L (Huang et al. 2022). In this report, we combined the D156R mutation from the low-temperature-tolerant variant and the E795L mutation to greatly enhance editing efficiency of LbCas12a (Fig. 1). Our results are consistent with a previous report that introducing mutations from a low-temperature-tolerant variant into a LbCas12a Ultra variant greatly enhanced editing efficiency of LbCas12a (Huang et al. 2022). The main difference with the previous report is that we used the variant harboring the single E795L mutation, whereas Huang et al. used the variant harboring the two mutations N527R and E795L. However, although E795L enhanced the editing efficiency, the mutation N527R showed the detrimental effect on LbCas12a activity, and the variant harboring the two mutations failed to show improved editing efficiency in human cells (Zhang et al. 2023). In the future, it will be interesting to compare the ttLbCas12a Ultra variant generated in this report to other highly active variants, such as Cas12a-Plus (Huang et al. 2022), hyper-Cas12a (Guo et al. 2022), iCas12a (Ma et al. 2022), and LbCas12a-RRV (Zhang et al. 2023) for use in plants. These comparisons will help generate more Cas12a variants with higher editing efficiency.
Since processing of tRNA-cRNAs or HH-crRNAs is not complete (Gao and Zhao 2014; ** of targeted mutations. Mol Plant 8:1431–1433. https://doi.org/10.1016/j.molp.2015.05.009 " href="/article/10.1007/s42994-024-00144-w#ref-CR17" id="ref-link-section-d68206792e1757">2015).
Data Availability
Annotated sequences of vectors described in this study and deep sequencing data are available from the corresponding author on reasonable request.
References
Banakar R, Schubert M, Collingwood M, Vakulskas C, Eggenberger AL, Wang K (2020) Comparison of CRISPR-Cas9/Cas12a ribonucleoprotein complexes for genome editing efficiency in the rice phytoene desaturase (OsPDS) gene. Rice 13:4. https://doi.org/10.1186/s12284-019-0365-z
Bernabe-Orts JM, Casas-Rodrigo I, Minguet EG, Landolfi V, Garcia-Carpintero V, Gianoglio S et al (2019) Assessment of Cas12a-mediated gene editing efficiency in plants. Plant Biotechnol J 17:1971–1984. https://doi.org/10.1111/pbi.13113
Blomme J, Develtere W, Kose A, Arraiza Ribera J, Brugmans C, Jaraba-Wallace J et al (2022) The heat is on: a simple method to increase genome editing efficiency in plants. BMC Plant Biol 22:142. https://doi.org/10.1186/s12870-022-03519-7
Chen PJ, Hussmann JA, Yan J, Knip** F, Ravisankar P, Chen PF et al (2021) Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184(5635–5652):e5629. https://doi.org/10.1016/j.cell.2021.09.018
Dong D, Ren K, Qiu X, Zheng J, Guo M, Guan X et al (2016) The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 532:522–526. https://doi.org/10.1038/nature17944
Gao X, Chen J, Dai X, Zhang D, Zhao Y (2016) An effective strategy for reliably isolating heritable and Cas9-free arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol 171:1794–1800. https://doi.org/10.1104/pp.16.00663
Gao Y, Zhao Y (2014) Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol 56:343–349. https://doi.org/10.1111/jipb.12152
Guo LY, Bian J, Davis AE, Liu P, Kempton HR, Zhang X et al (2022) Multiplexed genome regulation in vivo with hyper-efficient Cas12a. Nat Cell Biol 24:590–600. https://doi.org/10.1038/s41556-022-00870-7
Huang H, Huang G, Tan Z, Hu Y, Shan L, Zhou J et al (2022) Engineered Cas12a-Plus nuclease enables gene editing with enhanced activity and specificity. BMC Biol 20:91. https://doi.org/10.1186/s12915-022-01296-1
Kurokawa S, Rahman H, Yamanaka N, Ishizaki C, Islam S, Aiso T et al (2021) A simple heat treatment increases SpCas9-mediated mutation efficiency in Arabidopsis. Plant Cell Physiol 62:1676–1686. https://doi.org/10.1093/pcp/pcab123
LeBlanc C, Zhang F, Mendez J, Lozano Y, Chatpar K, Irish VF, Jacob Y (2018) Increased efficiency of targeted mutagenesis by CRISPR/Cas9 in plants using heat stress. Plant J 93:377–386. https://doi.org/10.1111/tpj.13782
Lee K, Zhang Y, Kleinstiver BP, Guo JA, Aryee MJ, Miller J et al (2019) Activities and specificities of CRISPR/Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant Biotechnol J 17:362–372. https://doi.org/10.1111/pbi.12982
Li S, Zhang X, Wang W, Guo X, Wu Z, Du W et al (2018) Expanding the scope of CRISPR/Cpf1-mediated genome editing in rice. Mol Plant 11:995–998. https://doi.org/10.1016/j.molp.2018.03.009
Li S, Zhang Y, **a L, Qi Y (2020) CRISPR-Cas12a enables efficient biallelic gene targeting in rice. Plant Biotechnol J 18:1351–1353. https://doi.org/10.1111/pbi.13295
Liu P, Luk K, Shin M, Idrizi F, Kwok S, Roscoe B et al (2019a) Enhanced Cas12a editing in mammalian cells and zebrafish. Nucleic Acids Res 47:4169–4180. https://doi.org/10.1093/nar/gkz184
Liu Q, Wang C, Jiao X, Zhang H, Song L, Li Y et al (2019b) Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci China Life Sci 62:1–7. https://doi.org/10.1007/s11427-018-9402-9
Liu W, ** of targeted mutations. Mol Plant 8:1431–1433. https://doi.org/10.1016/j.molp.2015.05.009
Ma E, Chen K, Shi H, Stahl EC, Adler B, Trinidad M et al (2022) Improved genome editing by an engineered CRISPR-Cas12a. Nucleic Acids Res 50:12689–12701. https://doi.org/10.1093/nar/gkac1192
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ et al (2020) Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 18:67–83. https://doi.org/10.1038/s41579-019-0299-x
Malzahn AA, Tang X, Lee K, Ren Q, Sretenovic S, Zhang Y et al (2019) Application of CRISPR-Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol 17:9. https://doi.org/10.1186/s12915-019-0629-5
Merker L, Schindele P, Huang TK, Wolter F, Puchta H (2020) Enhancing in planta gene targeting efficiencies in Arabidopsis using temperature-tolerant CRISPR/LbCas12a. Plant Biotechnol J 18:2382–2384. https://doi.org/10.1111/pbi.13426
Schindele P, Merker L, Schreiber T, Prange A, Tissier A, Puchta H (2023) Enhancing gene editing and gene targeting efficiencies in Arabidopsis thaliana by using an intron-containing version of ttLbCas12a. Plant Biotechnol J 21:457–459. https://doi.org/10.1111/pbi.13964
Schindele P, Puchta H (2020) Engineering CRISPR/LbCas12a for highly efficient, temperature-tolerant plant gene editing. Plant Biotechnol J 18:1118–1120. https://doi.org/10.1111/pbi.13275
Su H, Wang Y, Xu J, Omar AA, Grosser JW, Calovic M et al (2023) Generation of the transgene-free canker-resistant Citrus sinensis using Cas12a/crRNA ribonucleoprotein in the T0 generation. Nat Commun 14:3957. https://doi.org/10.1038/s41467-023-39714-9
Tang X, Lowder LG, Zhang T, Malzahn AA, Zheng X, Voytas DF et al (2017) A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants 3:17103. https://doi.org/10.1038/nplants.2017.103
Toth E, Varga E, Kulcsar PI, Kocsis-Jutka V, Krausz SL, Nyeste A et al (2020) Improved LbCas12a variants with altered PAM specificities further broaden the genome targeting range of Cas12a nucleases. Nucleic Acids Res 48:3722–3733. https://doi.org/10.1093/nar/gkaa110
Wang M, Mao Y, Lu Y, Tao X, Zhu JK (2017) Multiplex gene editing in rice using the CRISPR-Cpf1 system. Mol Plant 10:1011–1013. https://doi.org/10.1016/j.molp.2017.03.001
Wang W, Tian B, Pan Q, Chen Y, He F, Bai G et al (2021) Expanding the range of editable targets in the wheat genome using the variants of the Cas12a and Cas9 nucleases. Plant Biotechnol J 19:2428–2441. https://doi.org/10.1111/pbi.13669
Wolter F, Puchta H (2019) In planta gene targeting can be enhanced by the use of CRISPR/Cas12a. Plant J 100:1083–1094. https://doi.org/10.1111/tpj.14488
**e K, Minkenberg B, Yang Y (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci USA 112:3570–3575. https://doi.org/10.1073/pnas.1420294112
Xu R, Qin R, Li H, Li D, Li L, Wei P, Yang J (2017) Generation of targeted mutant rice using a CRISPR-Cpf1 system. Plant Biotechnol J 15:713–717. https://doi.org/10.1111/pbi.12669
Xu R, Qin R, Li H, Li J, Yang J, Wei P (2019) Enhanced genome editing in rice using single transcript unit CRISPR-LbCpf1 systems. Plant Biotechnol J 17:553–555. https://doi.org/10.1111/pbi.13028
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P et al (2015) Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163:759–771. https://doi.org/10.1016/j.cell.2015.09.038
Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM et al (2017) Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 35:31–34. https://doi.org/10.1038/nbt.3737
Zhang L, Li G, Zhang Y, Cheng Y, Roberts N, Glenn SE et al (2023) Boosting genome editing efficiency in human cells and plants with novel LbCas12a variants. Genome Biol 24:102. https://doi.org/10.1186/s13059-023-02929-6
Zhang L, Zuris JA, Viswanathan R, Edelstein JN, Turk R, Thommandru B et al (2021) AsCas12a ultra nuclease facilitates the rapid generation of therapeutic cell medicines. Nat Commun 12:3908. https://doi.org/10.1038/s41467-021-24017-8
Zhang Q, Zhang Y, Chai Y (2022) Optimization of CRISPR/LbCas12a-mediated gene editing in Arabidopsis. PLoS ONE 17:e0265114. https://doi.org/10.1371/journal.pone.0265114
Zhong Z, Zhang Y, You Q, Tang X, Ren Q, Liu S et al (2018) Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and altered PAM sites. Mol Plant 11:999–1002. https://doi.org/10.1016/j.molp.2018.03.008
Zhou J, Liu G, Zhao Y, Zhang R, Tang X, Li L et al (2023) An efficient CRISPR-Cas12a promoter editing system for crop improvement. Nat Plants 9:588–604. https://doi.org/10.1038/s41477-023-01384-2
Acknowledgements
We thank Dr. Yunde Zhao from University of California San Diego for providing us with pHDE-35SCas9-mCherry.
Funding
This work was supported by grants from the National Key Research and Development Program of China (grant no. 2023YFD1202905).
Author information
Authors and Affiliations
Contributions
QC and XW conceived and designed the research. CX, DQ, JW, WS, ZC, YL, YJ, and YC conducted the experiments and analyzed the data. QC, CX, and XW wrote the manuscript. All authors read and approved the final version.