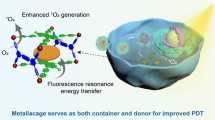
Abstract
The combination of photodynamic therapy (PDT) and chemodynamic therapy (CDT) in tumor treatment has attracted considerable attention. However, tumor hypoxia and glutathione (GSH) overproduction in the tumor tissue restricted the progress of their applications. Herein, a Mn-porphyrinic metal–organic framework (Mn-TCPP) was constructed by the one-pot method and further used for immobilizing glucose oxidase (GOx) to obtain GOx@Mn-TCPP. GOx would rapidly exhaust endogenous glucose into hydrogen peroxide (H2O2) and gluconic acid, thus shutting off the energy supply of tumor cells for starvation treatment. Mn-TCPP catalyzed H2O2 to produce oxygen, regulating the hypoxic tumor microenvironment and in turn improving 1O2 generation under laser irradiation. Interestingly, Mn-TCPP can reduce reactive oxygen species consumption owing to the redox reaction between Mn3+ and GSH, thus greatly enhancing PDT. Furthermore, benefiting from GOx-mediated starvation therapy, Mn2+ produced by Mn3+ reduction can react with sufficient intracellular H2O2 to generate ·OH with high cytotoxicity through a Fenton-like reaction. After treatment by GOx@Mn-TCPP under laser irradiation in vitro, the cell viability of 4T1 and A549 tumor cells reached to 20%, reflecting GOx@Mn-TCPP could give full play to the advantages of PDT/CDT/starvation therapy. The results in vivo demonstrated that GOx@Mn-TCPP mediated synergistic cascade therapy could significantly inhibit tumor growth and improve the therapeutic effect.







Similar content being viewed by others
Data availability
The data generated during and/or analyszed in this article are available from the corresponding author on the reasonable request.
References
Yang G, Mu X, Pan X, Tang Y, Yao Q, Wang Y, Jiang F, Du F, **e J, Zhou X, Yuan X. Ligand engineering of Au44 nanoclusters for NIR-II luminescent and photoacoustic imaging-guided cancer photothermal therapy. Chem Sci. 2023;14:4308. https://doi.org/10.1039/D2SC05729H.
Yang G, Pan X, Feng W, Yao Q, Jiang F, Du F, Zhou X, **e J, Yuan X. Engineering Au44 nanoclusters for NIR-II luminescence imaging-guided photoactivatable cancer immunotherapy. ACS Nano. 2023;17(16):15605. https://doi.org/10.1021/acsnano.3c02370.
Shi J. Active reactive oxygen species removal by movable hollow ROS-nanoscavengers. Chem. 2019;5(9):2285. https://doi.org/10.1016/j.chempr.2019.08.001.
Gao F, Shao T, Yu Y, **ong Y, Yang L. Surface-bound reactive oxygen pecies generating nanozymes for selective antibacterial action. Nat Commun. 2021;12(1):1. https://doi.org/10.1038/s41467-021-20965-3.
Wang LM, Liu WY, Hu ML, Yao JS, Wang P, Liu JH, He M, Gao Y, Li ZX. Rare earth-based MOF@mesoporous silica nanoplatform for long-term and luminescence trackable chemotherapy.Rare Met. 2022;41(8):2701. https://doi.org/10.1007/s12598-022-01978-3.
Lin X, Wu H, Zhang J, Chen X, Gao X, Liu Y. Nucleic acid-MOF nanoparticle conjugates for NIR/ATP-driven synergetic photo-chemotherapy with hypoxia relief. Chem Eng J. 2024;480(15): 147865. https://doi.org/10.1016/j.cej.2023.147865.
Ling P, Cheng S, Chen N, Qian C, Gao F. Nanozyme-modified metal–organic frameworks with multienzymes activity as biomimetic catalysts and electrocatalytic interfaces. ACS Appl Mater Interfaces. 2020;12(15):17185. https://doi.org/10.1021/acsami.9b23147.
Chen W, He H, Jiao P, Han L, Li J, Wang X, Guo X. Metal–organic framework for hypoxia/ros/pH triple-responsive cargo release. Adv Healthc Mater. 2023;12(29):2301785. https://doi.org/10.1002/adhm.202301785.
Zhang Y, Wang F, Liu C, Wang Z, Kang L, Huang Y, Dong K, Ren J, Qu X. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018;12(1):651. https://doi.org/10.1021/acsnano.7b07746.
Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: tumour microenvironment-mediated fenton and fenton-like reactions. Angew Chem Int Ed. 2018;58(4):946. https://doi.org/10.1002/anie.201805664.
Wang X, Zhong X, Liu Z, Cheng L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today. 2020;35: 100946. https://doi.org/10.1016/j.nantod.2020.100946.
Lin L, Huang T, Song J, Ou X, Wang Z, Deng H, Tian R, Liu Y, Wang J, Liu Y, Yu G, Zhou Z, Wang S, Niu G, Yang H, Chen X. Synthesis of copper peroxide nanodots for H2O2 self-Supplying chemodynamic therapy. J Am Chem Soc. 2019;141(25):9937. https://doi.org/10.1002/anie.201805664.
Lin L, Song S, Song J, Ke L, Liu K, Zhou Y, Shen Z, Li Z, Yang J, Tang Z, Niu W, Yang G, Chen X. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed. 2018;57(18):4902. https://doi.org/10.1002/anie.201712027.
Ma B, Wang S, Liu F, Zhang S, Duan J, Li Z, Kong Y, Sang Y, Liu H, Bu W, Li L. Self-assembled copper–amino acid nanoparticles for in situ glutathione “and” H2O2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2018;141(2):849. https://doi.org/10.1021/jacs.8b08714.
Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun. 2015;6(1):1. https://doi.org/10.1038/ncomms9785.
Hu D, Zhong L, Wang M, Li H, Qu Y, Liu Q, Han R, Yuan L, Shi K, Peng J, Qian Z. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv Funct Mater. 2019;29(9):1806199. https://doi.org/10.1002/adfm.201806199.
Shi X, Yang W, Ma Q, Lu Y, Xu Y, Bian K, Liu F, Shi C, Wang H, Shi Y, Zhang B. Hemoglobin-mediated biomimetic synthesis of paramagnetic O2-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics. 2020;10(25):11607. https://doi.org/10.7150/thno.46228.
Chen Q, Chen J, Liang C, Feng L, Dong Z, Song X, Song G, Liu Z. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. J Controlled Release. 2017;263:79. https://doi.org/10.1016/j.jconrel.2016.11.006.
Zhang Y, Shen T, Kirillov A, Liu M, Tang W. NIR light/H2O2-triggered nanocomposites for a highly efficient and selective synergistic photodynamic and photothermal therapy against hypoxic tumor cells. Chem Commun. 2016;52(51):7939. https://doi.org/10.1039/c6cc02571d.
Sun X, Chen K, Liu Y, Zhang G, Shi M, Shi P, Zhang S. Metal-organic framework combined with CaO2 nanoparticles for enhanced and targeted photodynamic therapy. Nanoscale Adv. 2021;3(23):6669. https://doi.org/10.1039/d1na00610j.
Sun X, Zhang G, Ding X, Liu Y, Chen K, Shi P, Zhang S. A DNA functionalized metal–organic framework combined with magnesium peroxide nanoparticles: targeted and enhanced photodynamic therapy. Mater Chem Front. 2022;6(7):956. https://doi.org/10.1039/D1QM01475G.
Chen K, Sun X, Liu Y, Yang Y, Shi M, Yu J, Zhang S, Shi P. CeO2-decorated metal-organic framework for enhanced photodynamic therapy. Inorg Chem. 2022;61(41):16307. https://doi.org/10.1021/acs.inorgchem.2c02227.
Wang Y, Yin W, Ke W, Chen W, He C, Ge Z. Multifunctional polymeric micelles with amplified fenton reaction for tumor ablation. Biomacromol. 2018;19(6):1990. https://doi.org/10.1021/acs.biomac.7b01777.
Zhang HZ, Zhao HX, Chang WH, Liu XY, Chen P, Yu AQ, Aadil NC, Zhang YZ, Ni LB, Wang XQ, Wei YG. Hetero-bimetallic transition metal-substituted Krebs-type polyoxometalate with N-chelating ligand as anticancer agents. Tungsten.2023;5(2):225. https://doi.org/10.1007/s42864-023-00210-8.
Mu J, He L, Fan W, Tang W, Wang Z, Jiang C, Zhang D, Liu Y, Deng H, Zou J, Jacobson O, Qu J, Huang P, Chen X. Cascade reactions catalyzed by planar metal-organic framework hybrid architecture for combined cancer therapy. Small. 2020;16(42):2004016. https://doi.org/10.1002/smll.202004016.
Wang X, Zeng J, Zhang M, Zeng X, Zhang X. A versatile Pt-based core-shell nanoplatform as a nanofactory for enhanced tumor therapy. Adv Funct Mater. 2018;28(36):1801783. https://doi.org/10.1002/adfm.201801783.
Dong J, Wang SQ, Zhang XY, Huang ZQ, Zhang XD, Sun WY. Turn-on fluorescence detection of specific inorganic anions by Zr(IV)-MOF with amino-functional group. Tungsten. 2023;5(2):217. https://doi.org/10.1007/s42864-022-00198-7.
Zhou HJ, Kitagawa S. Metal–organic frameworks (MOFs). Chem Soc Rev. 2014;43(16):5415. https://doi.org/10.1039/C4CS90059F.
Furukawa H, Cordova K, O’Keeffe M, Yaghi OM. The chemistry and applications of metal-organic frameworks. Sci. 2013;341(6149):1230444. https://doi.org/10.1126/science.1230444.
Rieter WJ, Taylor KML, Lin W. Surface modification and functionalization of nanoscale metal-organic frameworks for controlled release and luminescence sensing. J Am Chem Soc. 2007;129(32):9852. https://doi.org/10.1021/ja073506r.
Bahari D, Babamiri B, Moradi K, Salimi A, Hallaj R. Graphdiyne nanosheet as a novel sensing platform for self-enhanced electrochemiluminescence of MOF enriched ruthenium (II) in the presence of dual co-reactants for detection of tumor marker. Biosens Bioelectron. 2022;195:113657. https://doi.org/10.1016/j.bios.2021.113657.
Biswas S, Lan Q, **e Y, Sun X, Wang Y. Label-free electrochemical immunosensor for ultrasensitive detection of carbohydrate antigen 125 based on antibody-immobilized biocompatible MOF-808/CNT. ACS Appl Mater Interfaces. 2021;13(2):3295. https://doi.org/10.1016/j.bios.2021.113657.
Min H, Wang J, Qi Y, Zhang Y, Han X, Xu Y, Xu J, Li Y, Chen L, Cheng K, Liu G, Yang N, Li Y, Nie G. Biomimetic metal-organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv Mater. 2019;31(15):1808200. https://doi.org/10.1002/adma.201808200.
Zhang Y, Wang Q, Chen G, Shi P. DNA-functionalized metal–organic framework: cell imaging, targeting drug delivery and photodynamic therapy. Inorg Chem. 2019;58(10):6593. https://doi.org/10.1021/acs.inorgchem.9b00734.
Dai H, Yan H, Dong F, Zhang L, Du N, Sun L, Li N, Yu G, Yang Z, Wang Y, Huang M. Tumor-targeted biomimetic nanoplatform precisely integrates photodynamic therapy and autophagy inhibition for collaborative treatment of oral cancer. Biomater Sci. 2022;10(6):1456. https://doi.org/10.1039/D1BM01780B.
Liu B, Liu Z, Lu X, Wu P, Sun Z, Chu H, Peng H. Controllable growth of drug-encapsulated metal-organic framework (MOF) on porphyrinic MOF for pdt/chemo-combined therapy. Mater Des. 2023;228: 111861. https://doi.org/10.1016/j.matdes.2023.111861.
Yang J, Yang YW. Metal–organic frameworks for biomedical applications. Small. 2020;16(10):1906846. https://doi.org/10.1002/smll.201906846.
Lawson HD, Walton SP, Chan C. Metal–organic frameworks for drug delivery: a design perspective. ACS Appl Mater Interfaces. 2021;13(6):7004. https://doi.org/10.1021/acsami.1c01089.
Lu K, He C, Lin W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J Am Chem Soc. 2014;136(48):16712. https://doi.org/10.1021/ja508679h.
Ranji-Burachaloo H, Karimi F, **e K, Fu Q, Gurr PA, Dunstan DE, Qiao GG. MOF-mediated destruction of cancer using the cell’s own hydrogen peroxide. ACS Appl Mater Interfaces. 2017;9(39):33599. https://doi.org/10.1021/acsami.7b07981.
Wan X, Song L, Pan W, Zhong H, Li N, Tang B. Tumor-targeted cascade nanoreactor based on metal–organic frameworks for synergistic ferroptosis–starvation anticancer therapy. ACS Nano. 2020;14(9):11017. https://doi.org/10.1021/acsnano.9b07789.
You Y, Xu D, Pan X, Ma X. Self-propelled enzymatic nanomotors for enhancing synergetic photodynamic and starvation therapy by self-accelerated cascade reactions. Appl Mater Today. 2019;16:508. https://doi.org/10.1016/j.apmt.2019.07.008.
Liu C, **ng J, Akakuru OU, Luo L, Sun S, Zou R, Yu Z, Fang Q, Wu A. Nanozymes-engineered metal-organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett. 2019;19(8):5674. https://doi.org/10.1021/acs.nanolett.9b02253.
Wang ZY, Mei J, Ni DQ, Li SM, Ye JM, Li SL, Wang YL, Liu Y. A nanoplatform self-assembled by coordination delivers siRNA for lung cancer therapy. Rare Met. 2023;42(5):1483. https://doi.org/10.1007/s12598-022-02185-w.
Liu X, Meng C, Ji GQ, Liu J, Zhu P, Qian JQ, Zhu SX, Zhang YN, Ling Y. Tumor microenvironment-activatable boolean logic supramolecular nanotheranostics based on a pillar arene for tumor hypoxia imaging and multimodal synergistic therapy. Mater Chem Front. 2021;15(5):5846. https://doi.org/10.1039/D1QM00411E.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (Nos. ZR2023MB139, ZR2023QB057), and the Key R&D Projects of Linyi City (2022022).
Author information
Authors and Affiliations
Contributions
**n-Ran Sun and Hao-Ming Yuan wrote the draft; **n-Ran Sun and Guo-Da Zhang collected the data; Chao Wang and Hao-Ming Yuan revised the paper, Shu-Juan Sun and Peng-Fei Shi contributed to conceived the idea of the study. All authors contributed to the writing and revisions. **n-Ran Sun and Hao-Ming Yuan contributed equally to this work and can be considered co-first authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, XR., Yuan, HM., Zhang, GD. et al. A Mn-porphyrinic metal–organic framework immobilizing glucose oxidase for combined photodynamic/chemodynamic/starvation therapy. Tungsten (2024). https://doi.org/10.1007/s42864-024-00283-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42864-024-00283-z