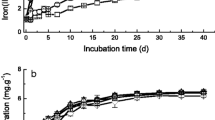
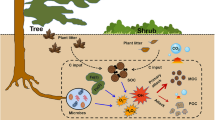
Abstract
Carbon materials (e.g., pyrogenic carbon (PyC)) are widely used in agricultural soils and can participate in various biogeochemical processes, including iron (Fe) cycling. In soils, Fe(II) species have been proposed as the main active contributor to produce reactive oxygen species (ROS), which are involved in various biogeochemical processes. However, the effects of PyC on the transformation of different Fe species in soils and the associated production of ROS are rarely investigated. This study examined the influence of PyC (pyrolyzed at 300–700 °C) on Fe(II)/Fe(III) cycling and hydroxyl radical (·OH) production during redox fluctuations of paddy soils. Results showed that the reduction of Fe(III) in soils was facilitated by PyC during anoxic incubation, which was ascribed to the increased abundance of dissimilatory Fe(III)-reducing microorganisms (biotic reduction) and the electron exchange capacity of PyC (abiotic reduction). During oxygenation, PyC and higher soil pH promoted the oxidation of active Fe(II) species (e.g., exchangeable and low-crystalline Fe(II)), which consequently induced higher yield of ·OH and further led to degradation of imidacloprid and inactivation of soil microorganisms. Our results demonstrated that PyC accelerated Fe(II)/Fe(III) cycling and ·OH production during redox fluctuations of paddy soils (especially those with low content of soil organic carbon), providing a new insight for remediation strategies in agricultural fields contaminated with organic pollutants.
Graphical Abstract

Highlights
-
Pyrogenic carbon (PyC) with high EEC promoted active Fe(II) formation in anoxic paddy soils through abiotic and biotic mechanisms.
-
Pyrolysis temperature affected the physiochemical properties of PyC, which regulated Fe cycling and ·OH production in soils.
-
The enhanced production of ·OH led to microbial inactivation and organic pollutant degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dynamic changes of redox potential have been widely observed during underground water recharge of unconfined river aquifers, water table fluctuations of lake sediments, and flooding-drainage cycles of cultivated lands (Du et al. 2021; Honma et al. 2016; Zhang et al. 2020a). The frequent redox fluctuations in these water-soil environments lead to intense interactions between reduced substances and oxygen (O2), which consequently induce the production of reactive oxygen species (ROS) (Chen et al. 2021b; Page et al. 2013; Yuan et al. 2017). Ubiquitous ROS production in redox oscillation events has arisen increasing attention and the potential roles of ROS in multiple biogeochemical processes have been proposed (Yu and Kuzyakov 2021). As one of the most reactive ROS, hydroxyl radical (·OH, E0 = 2.8 V vs. NHE) has critical impacts on the transformation of redox-active metals (Kong et al. 2015; Liu et al. 2022b), degradation of organic pollutants (** iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4(10):752–764" href="/article/10.1007/s42773-023-00236-8#ref-CR59" id="ref-link-section-d143775191e649">2006). In paddy fields, agricultural strategies such as flooding-drainage management and application of remediation agents can greatly influence microbial community structure and soil redox conditions (Huang et al. 2021a). To maintain agricultural production, amendments such as pyrogenic carbon (PyC) have been widely applied to paddy fields with several tons per hectare per year (Liu et al. 2022; Sun et al. 2017). EPR spectrum analysis revealed that PyC500 contained abundant carbon-centered persistent free radicals (1.09 × 106 spin g−1, g-factor of 2.0027, Additional file 1: Fig. S17e), which are also involved in electron transfer processes (Xu et al. 2016).
To distinguish the contribution of PyC (abiotic) and microorganisms (biotic) to Fe reduction, soils were sterilized by 1% (m/v) HgCl2 to eliminate microorganisms. Interestingly, despite soil microorganisms being eliminated, Fe reduction was observed in both CS and YT soils with the addition of PyC300 (1.44 and 1.17 g kg−1) and PyC500 (1.16 and 0.76 g kg−1) (Additional file 1: Fig. S18). These results indicated that PyC300 and PyC500 reduced Fe minerals abiotically, which was consistent with their high EDC (Additional file 1: Fig. S17a). Abiotic reduction of Fe minerals by PyC300, PyC500, and PyC700 contributed to 22.2%, 17.1%, and 4.0% of total Fe reduction in CS soil, and 19.7%, 11.4%, and 4.6% in YT soil, respectively (Additional file 1: Table S8). Abiotic reduction by different PyC resulted in the transformation of different Fe(II) species in soils (Additional file 1: Fig. S19). Specifically, PyC300 significantly promoted the formation of 5 M HCl-Fe(II), and PyC500 promoted the formation of 0.5 M HCl-Fe(II) and 5 M HCl-Fe(II). The formation of high-crystalline Fe(II) in the presence of PyC300 and PyC500 may be due to the reversible redox reactions between quinone and hydroquinone groups, which can transform low-crystalline Fe minerals to high-crystalline minerals through a dissolution-reprecipitation mechanism (Lian et al. 2022), higher redox potential of ·OH (E0 = 2.8 V) may favor the degradation of organic pollutants in soils (Yu and Kuzyakov 2021). Degradation of IMI in the YT and CS slurries was enhanced with the addition of PyC500, which was ascribed to the higher ·OH production as aforementioned (Fig. 1). Except for exogenous anthropogenic organic pollutants, the oxidation of soil organic carbon by reactive ·OH has also been proposed (Chen et al. 2021c). Assuming that 1 mol ·OH can effectively react with humic substances to produce 0.3 mol CO2 (Goldstone et al. 2002; Page et al. 2013), the enhanced ·OH production in CS- and YT-3%PyC500 slurries would result in 5.6–9.8 μmol L–1 more CO2 production after 8-h oxygenation.
4 Conclusion
Given the importance of PyC in participating in various biogeochemical processes, an investigation into the integrated effects of PyC on Fe cycling and ROS production in soil systems is required. Under the anoxic condition, PyC (especially PyC500 with high EEC) promoted the formation of active Fe (II) species mainly through abiotically reducing Fe minerals and enhancing the relative abundance of Fe(III)-reducing microorganisms, and therefore increased ·OH production during oxygenation. With PyC amendment, accelerated Fe(II) oxidation was consistent with the higher ·OH production rate, indicating that enhanced Fe(II)/Fe(III) cycling and ·OH production occurred. The produced ·OH was capable of inducing microbial inactivation and organic pollutant degradation during oxygenation processes. Overall, this study helped to better understand the critical roles of PyC in driving Fe(II)/Fe(III) cycling and ROS production under redox conditions. Given the significance of Fe(II)/Fe(III) cycling and ROS production, these processes consequently influence the mobility of toxic metals (e.g., arsenic), degradation of organic pollutants, and nutrient cycling (e.g., C, N, and P). As a frequently used functional material, the utility of PyC to increase the content of soil organic carbon should be examined during redox fluctuations in long-time scales due to the enhanced ·OH production and probable organic carbon decomposition.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request. Additional information, including chemicals, detailed experimental methods, and results of additional experiments in forms of figures and tables are provided in Additional file 1.
References
Bonneville S, Behrends T, Van Cappellen P (2009) Solubility and dissimilatory reduction kinetics of iron(III) oxyhydroxides: a linear free energy relationship. Geochim Cosmochim Acta 73(18):5273–5282
Chen C, Kukkadapu R, Sparks DL (2015) Influence of coprecipitated organic matter on Fe2+(aq)-catalyzed transformation of ferrihydrite: implications for carbon dynamics. Environ Sci Technol 49(18):10927–10936
Chen C, Shen Y, Li Y, Zhang W, Zhao F (2021a) Demethylation of the antibiotic methylarsenite is coupled to denitrification in anoxic paddy soil. Environ Sci Technol 55(22):15484–15494
Chen N, Fu Q, Wu T, Cui P, Fang G, Liu C, Chen C, Liu G, Wang W, Wang D, Wang P, Zhou D (2021b) Active iron phases regulate the abiotic transformation of organic carbon during redox fluctuation cycles of paddy soil. Environ Sci Technol 55(20):14281–14293
Chen N, Huang D, Liu G, Chu L, Fang G, Zhu C, Zhou D, Gao J (2021c) Active iron species driven hydroxyl radicals formation in oxygenation of different paddy soils: implications to polycyclic aromatic hydrocarbons degradation. Water Res 203:117484
Chen Z, Wei W, Chen H, Ni B (2022) Recent advances in waste-derived functional materials for wastewater remediation. Eco-Environ Health 1(2):86–104
Cismasu AC, Williams KH, Nico PS (2016) Iron and carbon dynamics during aging and reductive transformation of biogenic ferrihydrite. Environ Sci Technol 50(1):25–35
Dong Y, Sanford RA, Boyanov MI, Flynn TM, O’Loughlin EJ, Kemner KM, George S, Fouke KE, Li S, Huang D, Li S, Fouke BW (2020) Controls on iron reduction and biomineralization over broad environmental conditions as suggested by the firmicutes Orenia metallireducens strain Z6. Environ Sci Technol 54(16):10128–10140
Du H, Cao Y, Li Z, Li L, Xu H (2021) Formation and mechanisms of hydroxyl radicals during the oxygenation of sediments in Lake Poyang. China Water Res 202:117442
Fang L, Xu L, Deng J, Gao S, Huang L (2021) Induced generation of hydroxyl radicals from green rust under oxic conditions by iron-phosphate complexes. Chem Eng J 414:128780
Feng D, Lü J, Guo S, Li J (2021) Biochar enhanced the degradation of organic pollutants through a Fenton process using trace aqueous iron. J Environ Chem Eng 9(1):104677
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61(20):14095–14107
Gao B, Chen Q, Liu K, Li F, Fang L, Zhu Z, Tran MT, Peng J (2022) Biogeochemical Fe(II) generators as a new strategy for limiting Cd uptake by rice and its implication for agricultural sustainability. Sci Total Environ 820:153306
Garg S, Jiang C, Waite TD (2018) Impact of pH on iron redox transformations in simulated freshwaters containing natural organic matter. Environ Sci Technol 52(22):13184–13194
Goldstone JV, Pullin MJ, Bertilsson S, Voelker BM (2002) Reactions of hydroxyl radical with humic substances: bleaching, mineralization, and production of bioavailable carbon substrates. Environ Sci Technol 36(3):364–372
Hong Z, Li F, Borch T, Shi Q, Fang L (2023) Incorporation of Cu into goethite stimulates oxygen activation by surface-bound Fe(II) for enhanced As(III) oxidative transformation. Environ Sci Technol 57(5):2162–2174
Honma T, Ohba H, Kaneko-Kadokura A, Makino T, Nakamura K, Katou H (2016) Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ Sci Technol 50(8):4178–4185
Huang H, Ji X, Cheng L, Zhao F, Wang P (2021a) Free radicals produced from the oxidation of ferrous sulfides promote the remobilization of cadmium in paddy soils during drainage. Environ Sci Technol 55(14):9845–9853
Huang J, Jones A, Waite TD, Chen Y, Huang X, Rosso KM, Kappler A, Mansor M, Tratnyek PG, Zhang H (2021b) Fe(II) redox chemistry in the environment. Chem Rev 121(13):8161–8233
Hudson JM, Luther GW, Chin Y (2022) Influence of organic ligands on the redox properties of Fe(II) as determined by mediated electrochemical oxidation. Environ Sci Technol 56(12):9123–9132
Jiang B, Duan D, Gao L, Zhou M, Fan K, Tang Y, ** J, Bi Y, Tong Z, Gao GF, **e N, Tang A, Nie G, Liang M, Yan X (2018) Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat Protoc 13(7):1506–1520
Jones A, Collins RN, Rose J, Waite TD (2009) The effect of silica and natural organic matter on the Fe(II)-catalysed transformation and reactivity of Fe(III) minerals. Geochim Cosmochim Acta 73(15):4409–4422
Kappler A, Wuestner ML, Ruecker A, Harter J, Halama M, Behrens S (2014) Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ Sci Technol Lett 1(8):339–344
Kappler A, Bryce C, Mansor M, Lueder U, Byrne JM, Swanner ED (2021) An evolving view on biogeochemical cycling of iron. Nat Rev Microbiol 19(6):360–374
Katsoyiannis IA, Ruettimann T, Hug SJ (2008) pH dependence of fenton reagent generation and As(III) oxidation and removal by corrosion of zero valent iron in aerated water. Environ Sci Technol 42(19):7424–7430
Klupfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48(10):5601–5611
Kong L, Hu X, He M (2015) Mechanisms of Sb(III) oxidation by pyrite-induced hydroxyl radicals and hydrogen peroxide. Environ Sci Technol 49(6):3499–3505
Kugler S, Cooper RE, Wegner C-E, Mohr JF, Wichard T, Kusel K (2019) Iron-organic matter complexes accelerate microbial iron cycling in an iron-rich fen. Sci Total Environ 646:972–988
Li L, Qu Z, Wang B, Qu D (2017) Dynamics of the abundance and structure of metabolically active Clostridium community in response to glucose additions in flooded paddy soils: closely correlated with hydrogen production and Fe(III) reduction. J Soils Sediments 17(6):1727–1740
Lian F, Gu S, Han Y, Wang Z, **ng B (2022) Novel insights into the impact of nano-biochar on composition and structural transformation of mineral/nano-biochar heteroaggregates in the presence of root exudates. Environ Sci Technol 56(13):9816–9825
Liptzin D, Silver WL (2009) Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical forest soil. Soil Biol Biochem 41(8):1696–1702
Liu D, Dong H, Bishop ME, Wang H, Agrawal A, Tritschler S, Eberl DD, **e S (2011) Reduction of structural Fe(III) in nontronite by methanogen Methanosarcina barkeri. Geochim Cosmochim Acta 75(4):1057–1071
Liu J, Li S, Yue S, Tian J, Chen H, Jiang H, Siddique KHM, Zhan A, Fang Q, Yu Q (2021) Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 396:115086
Liu K, Li F, Pang Y, Fang L, Hocking R (2022a) Electron shuttle-induced oxidative transformation of arsenite on the surface of goethite and underlying mechanisms. J Hazard Mater 425:127780
Liu W, Liu J, Zhou P, Dahlgren RA, Wang X (2022b) Mechanisms for hydroxyl radical production and arsenic removal in sulfur-vacancy greigite (Fe3S4). J Colloid Interface Sci 606:688–695
Liu Y, Wang M, Yin S, **e L, Qu X, Fu H, Shi Q, Zhou F, Xu F, Tao S, Zhu D (2022c) Comparing photoactivities of dissolved organic matter released from rice straw-pyrolyzed biochar and composted rice straw. Environ Sci Technol 56(4):2803–2815
Lu Y, Hu Y, Tang L, **e Q, Liu Q, Zhong L, Fu L, Fan C (2021) Effects and mechanisms of modified biochars on microbial iron reduction of Geobacter sulfurreducens. Chemosphere 283:130983
Mopper K, Zhou X (1990) Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science 250(4981):661–664
Page SE, Kling GW, Sander M, Harrold KH, Logan JR, McNeill K, Cory RM (2013) Dark formation of hydroxyl radical in arctic soil and surface waters. Environ Sci Technol 47(22):12860–12867
Park B, Dempsey BA (2005) Heterogeneous oxidation of Fe(II) on ferric oxide at neutral pH and a low partial pressure of O2. Environ Sci Technol 39(17):6494–6500
Pedersen HD, Postma D, Jakobsen R, Larsen O (2005) Fast transformation of iron oxyhydroxides by the catalytic action of aqueous Fe(II). Geochim Cosmochim Acta 69(16):3967–3977
Roden EE, Kappler A, Bauer I, Jiang J, Paul A, Stoesser R, Konishi H, Xu H (2010) Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat Geosci 3(6):417–421
Seo J, Lee H, Lee H, Kim H, Lee J, Kim HS, Lee C (2015) Enhanced production of reactive oxidants by Fenton-like reactions in the presence of carbon materials. Chem Eng J 273:502–508
Si D, Wu H, Yang M, Fan T, Wang D, Chen L, Zhu C, Fang G, Wu S, Zhou D (2022) Linking pyrogenic carbon redox property to arsenite oxidation: impact of N-do** and pyrolysis temperature. J Hazard Mater 445:130477
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22(1):5–34
Stern N, Mejia J, He S, Yang Y, Ginder-Vogel M, Roden EE (2018) Dual role of humic substances as electron donor and shuttle for dissimilatory iron reduction. Environ Sci Technol 52(10):5691–5699
Sun T, Levin BDA, Guzman JJL, Enders A, Muller DA, Angenent LT, Lehmann J (2017) Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat Commun 8(1):14873
Sun T, Levin BDA, Schmidt MP, Guzman JJL, Enders A, Martínez CE, Muller DA, Angenent LT, Lehmann J (2018) Simultaneous quantification of electron transfer by carbon matrices and functional groups in pyrogenic carbon. Environ Sci Technol 52(15):8538–8547
Sun T, Guzman JJL, Seward JD, Enders A, Yavitt JB, Lehmann J, Angenent LT (2021) Suppressing peatland methane production by electron snorkeling through pyrogenic carbon in controlled laboratory incubations. Nat Commun 12(1):4119
Tan M, Liu S, Chen N, Li Y, Ge L, Zhu C, Zhou D (2022) Hydroxyl radicals induced mineralization of organic carbon during oxygenation of ferrous mineral-organic matter associations: adsorption versus coprecipitation. Sci Total Environ 816:151667
Thompson A, Chadwick OA, Rancourt DG, Chorover J (2006) Iron-oxide crystallinity increases during soil redox oscillations. Geochim Cosmochim Acta 70(7):1710–1727
Tian S, Wang L, Liu Y, Ma J (2020) Degradation of organic pollutants by ferrate/biochar: enhanced formation of strong intermediate oxidative iron species. Water Res 183:116054
Tong M, Yuan S, Ma S, ** M, Liu D, Cheng D, Liu X, Gan Y, Wang Y (2016) Production of abundant hydroxyl radicals from oxygenation of subsurface sediments. Environ Sci Technol 50(1):214–221
Voinov MA, Pagán JOS, Morrison E, Smirnova TI, Smirnov AI (2011) Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J Am Chem Soc 133(1):35–41
Wang N, Xue X, Juhasz AL, Chang Z, Li H (2017) Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environ Pollut 220:514–522
Wang K, Jia R, Li L, Jiang R, Qu D (2020) Community structure of Anaeromyxobacter in Fe(III) reducing enriched cultures of paddy soils. J Soils Sediments 20(3):1621–1631
Wang D, Huang D, Wu S, Fang G, Zhu F, Chen N, Liu S, Zhu C, Zhou D (2021) Pyrogenic carbon initiated the generation of hydroxyl radicals from the oxidation of sulfide. Environ Sci Technol 55(9):6001–6011
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pum** iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4(10):752–764
Winkler P, Kaiser K, Thompson A, Kalbitz K, Fiedler S, Jahn R (2018) Contrasting evolution of iron phase composition in soils exposed to redox fluctuations. Geochim Cosmochim Acta 235:89–102
Wu S, Wang D, Liu C, Fang G, Sun T, Cui P, Yan H, Wang Y, Zhou D (2021) Pyridinic- and pyrrolic nitrogen in pyrogenic carbon improves electron shuttling during microbial Fe(III) reduction. ACS Earth Space Chem 5(4):900–909
**e W, Zhang P, Liao W, Tong M, Yuan S (2021) Ligand-enhanced electron utilization for trichloroethylene degradation by ·OH during sediment oxygenation. Environ Sci Technol 55(10):7044–7051
**n D, **an M, Chiu PC (2019) New methods for assessing electron storage capacity and redox reversibility of biochar. Chemosphere 215:827–834
Xu S, Adhikari D, Huang R, Zhang H, Tang Y, Roden E, Yang Y (2016) Biochar-facilitated microbial reduction of hematite. Environ Sci Technol 50(5):2389–2395
Xu Z, Yu Y, Xu X, Tsang DCW, Yao C, Fan J, Zhao L, Qiu H, Cao X (2022) Direct and indirect electron transfer routes of chromium(VI) reduction with different crystalline ferric oxyhydroxides in the presence of pyrogenic carbon. Environ Sci Technol 56(3):1724–1735
Yang F, Zhao L, Gao B, Xu X, Cao X (2016) The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ Sci Technol 50(5):2264–2271
Yang Z, Sun T, Kleindienst S, Straub D, Kretzschmar R, Angenent LT, Kappler A (2021) A coupled function of biochar as geobattery and geoconductor leads to stimulation of microbial Fe(III) reduction and methanogenesis in a paddy soil enrichment culture. Soil Biol Biochem 163:108446
Yu G, Kuzyakov Y (2021) Fenton chemistry and reactive oxygen species in soil: abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci Rev 214:103525
Yu C, Zhang Y, Lu Y, Qian A, Zhang P, Cui Y, Yuan S (2021a) Mechanistic insight into humic acid-enhanced hydroxyl radical production from Fe(II)-bearing clay mineral oxygenation. Environ Sci Technol 55(19):13366–13375
Yu W, Lawrence NC, Sooksa-nguan T, Smith SD, Tenesaca C, Howe AC, Hall SJ (2021b) Microbial linkages to soil biogeochemical processes in a poorly drained agricultural ecosystem. Soil Biol Biochem 156:108228
Yu W, Chu C, Chen B (2022) Enhanced microbial ferrihydrite reduction by pyrogenic carbon: impact of graphitic structures. Environ Sci Technol 56(1):239–250
Yuan X, Nico PS, Huang X, Liu T, Ulrich C, Williams KH, Davis JA (2017) Production of hydrogen peroxide in groundwater at Rifle. Colorado Environ Sci Technol 51(14):7881–7891
Zhang P, Yuan S, Liao P (2016) Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim Cosmochim Acta 172:444–457
Zhang N, Bu X, Li Y, Zhang Y, Yuan S, Wen Z, Tong M, Lin L (2020a) Water table fluctuations regulate hydrogen peroxide production and distribution in unconfined aquifers. Environ Sci Technol 54(8):4942–4951
Zhang P, Yuan S, Chen R, Bu X, Tong M, Huang Q (2020b) Oxygenation of acid sulfate soils stimulates CO2 emission: roles of acidic dissolution and hydroxyl radical oxidation. Chem Geol 533:119437
Zhao Q, Dunham-Cheatham S, Adhikari D, Chen C, Patel A, Poulson SR, Obrist D, Verburg PSJ, Wang X, Roden ER, Thompson A, Yang Y (2020) Oxidation of soil organic carbon during an anoxic-oxic transition. Geoderma 377:114584
Zhao Y, **ang W, Huang C, Liu Y, Tan Y (2021) Production of hydroxyl radicals following water-level drawdown in peatlands: a new induction mechanism for enhancing laccase activity in carbon cycling. Soil Biol Biochem 156:108241
Zhou G, Yang X, Li H, Marshall CW, Zheng B, Yan Y, Su J, Zhu Y (2016) Electron shuttles enhance anaerobic ammonium oxidation coupled to iron(III) reduction. Environ Sci Technol 50(17):9298–9307
Zhou G, Yang X, Marshall CW, Li H, Zheng B, Yan Y, Su J, Zhu Y (2017) Biochar addition increases the rates of dissimilatory iron reduction and methanogenesis in ferrihydrite enrichments. Front Microbiol 8:589
Zhu C, Xue C, Huang M, Zhu F, Fang G, Wang D, Liu S, Chen N, Wu S, Zhou D (2022) Rapid As(III) oxidation mediated by activated carbons: reactive species vs. direct oxidation. Sci Total Environ 822:153536
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (No. 42130707, 42107382, 42022049) and the Natural Science Foundation of Jiangsu Province (BK20200323).
Author information
Authors and Affiliations
Contributions
DZ and NC conceived the research idea. DH designed and performed laboratory analyses. YL, CG, XW, and DW assisted sample collections and analytical tools. CZ and GF assisted with data interpretation and mechanism discussion. DH produced the first draft of the manuscript, and all authors discussed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Baoshan ** of the pyrogenic carbon (PyC) in (a) CS-3%PyC300, (b) CS-3%PyC500, (c) CS-3%PyC700, (d) YT-3%PyC300, (e) YT-3%PyC500, and (f) YT-3%PyC700 anoxic slurries.Fig. S10. Effects of 2,2′-bipyridine (BPY), 1 mM nitrotetrazolium blue chloride (NBT), and 4000 U L–1 catalase (CAT) on hydroxyl radical (•OH) production in Changsha (CS) slurries (a) without Pyrogenic carbon (PyC) and with (b) 3%PyC300, (c) 3%PyC500, (d) 3%PyC700 during 8-h oxygenation. Fig. S11. The effect of γ irradiation on •OH production in 40-day anoxic incubated Changsha (CS) slurries.Fig. S12. (a) Cumulative •OH generated by pyrogenic carbon (PyC) or reduced pyrogenic carbon (rPyC) under oxic condition at pH 7 (10 mM phosphate buffer). Fig. S13. Correlation analyses between cumulative •OH and total extractable Fe(II) in (a) Changsha (CS) and (b) Yingtan (YT) slurries. Fig. S14. Changes of extractable Fe(II) species in Changsha (CS) slurries without (control) and with 1% (w/w) pyrogenic carbon (PyC300, PyC500, and PyC700) during 8-h oxygenation.Fig. S15. Variation of soil suspension pH of (a) Changsha (CS) and (b) Yingtan (YT) slurries during 8-h oxygenation.Fig. S16. Changes of Fe species in Changsha (CS) and Yingtan (YT) slurries during 8-h oxygenation.Fig. S17. Physicochemical properties of pyrogenic carbon. (a) Electron donating capacity (EDC) and electron accepting capacity (EAC); (b) FTIR spectra; (c) Raman spectra; (d) electrical conductivity; (e) EPR spectra; (f) peroxidase-like activity.Fig. S18. Content of total Fe(II) in sterilized Changsha (CS) and Yingtan (YT) soils without (control) and with 3% (w/w) pyrogenic carbon (PyC300, PyC500, and PyC700) during 20-day anoxic incubation.Fig. S19. Dynamic changes of (a, e) CaCl2-Fe(II), (b, f) 0.5 M HCl-Fe(II), (c, g) 5M HCl-Fe(II), and (d, h) HF-Fe(II) of sterilized Changsha (CS) and Yingtan (YT) paddy soil without (control) and with 3% (w/w) pyrogenic carbon (PyC300, PyC500, and PyC700) during 20-day anoxic incubation.Fig. S20. Content of total Fe(II) in sterilized Changsha (CS) and Yingtan (YT) soils without (control) and with 3% (w/w) reduced pyrogenic carbon (rPyC300, rPyC500, and rPyC700) during 20-day anoxic incubation. Fig. S21. Dynamic changes of (a, e) CaCl2-Fe(II), (b, f) 0.5 M HCl-Fe(II), (c, g) 5 M HCl-Fe(II), and (d, h) HF-Fe(II) of sterilized Changsha (CS) and Yingtan (YT) paddy soil during a 20-day anoxic incubation without (control) and with 3% (w/w) reduced pyrogenic carbon (rPyC300, rPyC500, and rPyC700).Fig. S22. Pathway of abiotic formation of different Fe(II) species in the presence of pyrogenic carbon (PyC300, PyC500, and PyC700) in soil.Fig. S23. Content of total extractable Fe(II) in Chongqing (CQ), Hebei (HB), and Sichuan (SC) slurries without (control) and with 3% (w/w) PyC500 after 20-day anoxic incubation.Fig. S24. Contents of (a) CaCl2-Fe(II), (b) 0.5 M HCl-Fe(II), (c) 5 M HCl-Fe(II), and (d) HF-Fe(II)) in Chongqing (CQ), Hebei (HB), and Sichuan (SC) slurries without (control) and with 3% (w/w) PyC500 after 20-day anoxic incubation.Fig. S25. (a) Microbial compositions of Yingtan (YT) slurries.Fig. S26. Correlation analyses between initial content of 0.5 or 5 M HCl-Fe(II), consumed 0.5 or 5 M HCl-Fe(II) and cumulative •OH. Fig. S27. Instantaneous H2O2 concentration during oxygenation.Fig. S28. Peroxidase-like activities of the Changsha (CS) and Yingtan (YT) slurries. Fig. S29. Concentration of dissolved organic matter (DOM). Fig. S30. Oxygenation of 1 mM Fe2+ and cumulative •OH production with pyrogenic carbon (PyC) or reduced pyrogenic carbon (rPyC) at pH 6 (20 mM MES buffer). Fig. S31. Physicochemical properties of reduced pyrogenic carbon. Fig. S32. The XPS O1s spectra of pyrogenic carbon. Fig. S33. Content of total extractable Fe(II) in CS slurries and (b) YT slurries. Fig. S34. •OH production in Yingtan (YT) slurries without (control) and with 3% (w/w) reduced pyrogenic carbon (rPyC300, rPyC500, and rPyC700) during 8-h oxygenation. Fig. S35. Changes of extractable Fe(II) species (CaCl2-Fe(II), 0.5 M HCl-Fe(II), 5 M HCl-Fe(II), and HF-Fe(II) in (a–d) Changsha (CS) and (e–h) Yingtan (YT) slurries without (control) and with 3% (w/w) reduced pyrogenic carbon (rPyC300, rPyC500, and rPyC700) during 8-h oxygenation.Fig. S36. The correlation heatmap of physicochemical properties of pyrogenic carbons with the fitted kinetic value kobs of •OH production. The physicochemical properties include electrical conductivity (EC), electron accepting capacity (EAC), electron donating capacity (EDC), electron exchange capacity (EEC, sum of EAC and EDC), persistent free radical concentrations (PFRs), the content of oxygen, and the content of oxygen-containing functional groups (i.e., C=O, C–O–C, and C–OH) (*p ≤ 0.05).Fig. S37. Numbers of living bacteria in (a) Changsha (CS) slurries and (b) Yingtan (YT) slurries after 4-h reaction with and without 100 mM ethanol. Anoxic controls were conducted in anoxic glovebox. Fig. S38. Fluorescent microscopy images of stained cells in Changsha (CS) slurries (CS-control and CS-3%PyC500). (a, d) Before oxidation; (b, e) after 4-h oxidation; (c, f) after 4-h oxidation with addition of 100 mM ethanol to quench •OH.Fig. S39. Degradation of imidacloprid after 4-h anoxic incubation or 4-h oxygenation of Changsha (CS) and Yingtan (YT) slurries.