Abstract
This study investigated the anti-inflammatory activity of Lactiplantibacillus plantarum IDCC 3501 isolated from kimchi (Korean fermented food) and its safety. When lipopolysaccharide (LPS)-induced RAW 264.7 macrophages were treated with cell-free supernatant from L. plantarum IDCC 3501, the mRNA expression level of inflammatory markers (i.e., TNF-α, IL-1β, and IL-6) was significantly reduced. In addition, the decreased cell viability by LPS was recovered and NO production in LPS-induced cell was also decreased. For the safety assessment, the genes responsible for antibiotic resistance and virulence were not detected from the genome analysis of this strain. Consistent with this, minimal inhibitory concentrations against various antibiotics, biogenic amines, and d-lactate production, as well as enzymatic and hemolysis activities, indicated that L. plantarum IDCC 3501 did not produce any harmful compounds during fermentation. Furthermore, no acute toxicity and mortality were observed in a murine mouse model. Based on our findings, L. plantarum IDCC 3501 is safe and beneficial for human consumption.
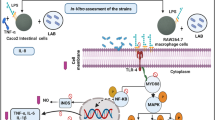
Graphical abstract



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its additional information files.
References
Siezen RJ, Tzeneva VA, Castioni A, Wels M, Phan HTK, Rademaker JLW, Starrenburg MJC, Kleerebezem M, Molenaar D, Van Hylckama Vlieg JET (2010) Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ Microbiol 12:758–773
Dinev T, Beev G, Tzanova M, Denev S, Dermendzhieva D, Stoyanova A (2018) Antimicrobial activity of Lactobacillus plantarum against pathogenic and food spoilage microorganisms: a review. Bulg J Vet Med 21:1–16
Tsai Y-T, Cheng P-C, Pan T-M (2014) Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl Microbiol Biotechnol 98:1–10
Duary RK, Bhausaheb MA, Batish VK, Grover S (2012) Anti-inflammatory and immunomodulatory efficacy of indigenous probiotic Lactobacillus plantarum Lp91 in colitis mouse model. Mol Biol Rep 39:4765–4775
Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A (2017) Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 9:555
Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D (2017) Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins 9:111–122
Jeong M, Kim JH, Yang H, Kang SD, Song S, Lee D, Lee JS, Yoon Park JH, Byun S, Lee KW (2019) Heat-killed Lactobacillus plantarum KCTC 13314BP enhances phagocytic activity and immunomodulatory effects via activation of MAPK and STAT3 pathways. J Microbiol Biotechnol 29:1248–1254
Shih R-H, Wang C-Y, Yang C-M (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8:77
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Rubin DC, Shaker A, Levin MS (2012) Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol 3:107
Le B, Yang SH (2018) Efficacy of Lactobacillus plantarum in prevention of inflammatory bowel disease. Toxicol Rep 5:314–317
Uchinaka A, Azuma N, Mizumoto H, Shiho N, Moeko M, Mamoru Y, Kiyoshi A, Yuki K, Yuichiro Y, Toyoaki M, Kohzo N (2018) Anti-inflammatory effects of heat-killed Lactobacillus plantarum L-137 on cardiac and adipose tissue in rats with metabolic syndrome. Sci Rep 8:1–20
Vijayalakshmi S, Adeyemi DE, Choi IY, Sultan G, Madar IH, Park M-K (2020) Comprehensive in silico analysis of lactic acid bacteria for the selection of desirable probiotics. LWT-Food Sci Technol 130:109617
Kim T-Y, Kim Y-H, Moon JS, Kwon H-S, Choi SK (2019) Complete genome sequence of probiotic Lactobacillus plantarum IDCC3501 isolated from kimchi. Korean J Microbiol 55:438–440
Chen L, Yang J, Yu J, Yao Z, Sun L, Shen Y, ** Q (2005) VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res 33:D325–D328
Clinical Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100 (ISBN 978-1-68440-066-9)
OECD (2008) Test No. 425: Acute oral toxicity: up-and-down procedure. OECD Guidelines for the Testing of Chemicals, Section 4, Part 425, Paris: OECD Publishing
De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, Pietrella D (2018) Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evi Based Complementy Altern Med 2018:1756308
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A (2018) Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol 75:105–114
Naka T, Nishimoto N, Kishimoto T (2002) The paradigm of IL-6: from basic science to medicine. Arthritis Res Ther 4:1–10
Je I-G, Lee D-G, Jeong D-G, Hong DH, Yoon J-M, Moon JS, Park SB (2018) The probiotic, ID-JPL934, attenuates dextran sulfate sodium-induced colitis in mice through inhibition of proinflammatory cytokines expression. J Med Food 21:858–865
Chang L, Zhang Z-Y, Dong K, Yuan J-P, Guo X-K (2009) Antibiotic resistance of probiotic strains of lactic acid bacteria isolated from marketed foods and drugs. Biome Environ Sci 22:401–412
Preena PG, Swaminathan TR, Kumar VJR, Singh ISB (2020) Antimicrobial resistance in aquaculture: a crisis for concern. Biologia 75:1497–1517
Zhou J, Pillidge C, Gopal P, Gill HS (2005) Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int J Food Microbiol 98:211–217
Temmerman R, Pot B, Huys G, Swings J (2003) Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol 81:1–10
Todorov SD, Perin LM, Carneiro BM, Rahal P, Holzapfel W, Nero LA (2017) Safety of Lactobacillus plantarum ST8Sh and its bacteriocin. Probiotics Antimicrob Proteins 9:334–344
Ruiz-Capillas C, Herrero AM (2019) Impact of biogenic amines on food quality and safety. Foods 8:62
Karovičová J, Kohajdová Z (2005) Biogenic amines in food. Chemical Papers 59:70–79
Barbieri F, Montanari C, Gardini F, Tabanelli G (2019) Biogenic amine production by lactic acid bacteria: a review. Foods 8:17
Gueimonde M, Frias R, Ouwehand A (2006) Assuring the continued safety of lactic acid bacteria used as probiotics. Biologia 61:755–760
Delgado S, O’sullivan E, Fitzgerald G, Mayo B (2007) Subtractive screening for probiotic properties of Lactobacillus species from the human gastrointestinal tract in the search for new probiotics. J Food Sci 72:M310-5
Awolade P, Cele N, Kerru N, Gummidi L, Oluwakemi E, Singh P (2020) Therapeutic significance of β-glucuronidase activity and its inhibitors: a review. Eur J Med Chem 187:111921
Son S-H, Jeon H-L, Yang S-J, Sim M-H, Kim Y-J, Lww N-K, Paik H-D (2018) Probiotic lactic acid bacteria isolated from traditional Korean fermented foods based on β-glucosidase activity. Food Sci Biotechnol 27:123–129
Bujnakova D, Strakova E, Kmet V (2014) In vitro evaluation of the safety and probiotic properties of lactobacilli isolated from chicken and calves. Anaerobe 29:118–127
Valan Arasu M, Jung M-W, Ilavenil S, Jane M, Kim D-H, Kee K-D, Park H-S, Hur T-Y, Choi G-J, Lim Y-C, Al-Dhabi NA, Choi K-C (2013) Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J App Microbiol 115:1172–1185
Ng SY, Koon SS, Padam BS, Chye FY (2015) Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang). CYTA-J FOOD 13:563–572
Nishida S, Ishii M, Nishiyama Y, Abe S, Ono Y, Sekimizu K (2017) Lactobacillus paraplantarum 11–1 isolated from rice bran pickles activated innate immunity and improved survival in a silkworm bacterial infection model. Front Microbiol 8:436
Funding
This work was supported by Ildong Bioscience and the National Research Foundation of Korea (NRF) funded by Korea government (Ministry of Science and ICT; Grant No. 2020R1C1C1005251).
Author information
Authors and Affiliations
Contributions
S-Y Yang, WY Bang, and SA Chae carried out the in vitro experiments and S-Y Yang drafted the manuscript. SA Chae and M Lee carried out the cell experiments. O–H Ban carried out the genetic studies, S-J Kim participated in the design of the study, and YH Jung and J Yang conceived the study and participated in its design and coordination, and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Luis Augusto Nero
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, SY., Chae, S.A., Bang, W.Y. et al. Anti-inflammatory potential of Lactiplantibacillus plantarum IDCC 3501 and its safety evaluation. Braz J Microbiol 52, 2299–2306 (2021). https://doi.org/10.1007/s42770-021-00603-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00603-2