Abstract
Highly hydrophobic surfaces exhibit a remarkable feature in the repellency of oil and water. However, the relatively complex preparation process, high costs, and harmful compounds have largely limited their applications. This research aim is to fabricate hydrophobic nonwoven fabrics with low-cost and nontoxic materials. Despite various wettable materials, nonwoven cotton fabric material bearing hydrophobic surfaces has been received significant attention. This is mainly owing to its easy handling, high flexibility, environment friendly, low cost, biodegradability, high efficiency, and easily scalable fabrication. In this study, a simple chemical modification method using hexadecyltrimethoxysilane (HDTMS) with ethanol which is a better method in comparison with other methods since it is an inexpensive, simple method, and offers an easy adjustment of chemical composition required for a surface to show hydrophobic behaviors. The wetting behavior of cotton samples was investigated by water contact angle measurement. The best result comes from 2 ml HDTMS with 40 ml ethanol at 60 °C. The result shows that the treated cotton fabrics exhibited excellent chemical stability and outstanding non-wettability with the WCA of 126 ± 2°. It also shows that standard oil and water repellency, which offers an opportunity to accelerate the large-scale production of hydrophobic textile materials for new industrial applications.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The quickly develo** worldwide vitality prerequisite has invigorated the fast advancement of oil and water repellency related ventures [1, 2]. Cotton fabrics are commonly used in our daily life because of their unique softness, breathability, warmth, comfort, and biodegradability properties [3, 4]. The natural and financial requests ask the need for practical materials that can adequately repellent oil and water [5]. Generally, the wettability of the materials for the particular appropriation of the oil and water repellency was requested to show super-hydrophobic properties [6, 7].
Cotton is broadly utilized in oil and water repellency for that they are modest and effortlessly achieved. However, they are unlikely to stay stable in the harsh chemical environment [8]. Great endeavors have been made to plan hydrophobic cotton or wipe, while commercially available super-hydrophobic cotton or sponge is still rare. Degradation of matrix materials, instability when applied to an extreme chemical environment, or mechanical friction restrict their practical application [9]. As indicated by the Wenzel and the Cassie–Baxter model, the presentation of an appropriate multi-scale harshness could make a perfect hydrophobic surface to be increasingly hydrophobic attributable to the air to be caught underneath the water bead as a pad [10,11,12]. There have been numerous reports demonstrating the likelihood to get ready hydrophobic materials by the mix of low surface vitality and appropriate harsh geology, just as the potential for oil and water repellency utilizing such materials. Due to the high importance of the field, the researchers are continuously putting their efforts to find out the effective methods or materials for oil and water repellency. Generally, the oil was expelled or disposed of from the water by utilizing the air buoyancy, skimming, burning, electric field, ultrasonic division, and oil corruption with the assistance of organisms [13, 14].
Nonwoven fabrics are extensively characterized as sheet or web structures fortified together by snaring fiber or fibers (and by puncturing films) precisely, thermally or artificially. They are level or tufted permeable sheets that are made straightforwardly from discrete strands, liquid plastic or plastic film. They are not made by weaving or sewing and do not require changing over the strands to yarn. Nonwoven fabric is a texture like material produced using staple fiber and long strands, reinforced together by compound, mechanical, warmth or dissolvable treatment. Cotton nonwovens contained 100% cotton fiber or cotton mix. As an all-common and hypo-allergenic fiber, cotton gives the perfect substance to earth inviting nonwovens [15]. Nonwoven cotton includes the qualities of unrivaled ingestion and discharge, with a high level of solace and non-abrasiveness. Nonwoven cotton textures give explicit capacities, for example, sponginess, fluid repellence, versatility, stretch, delicate quality, quality, fire retardancy, wash capacity, padding, warm protection, acoustic protection, filtration, use as a bacterial obstruction and sterility [16, 17].
The conventional strategies are experiencing low productivity, poor selectivity, significant expense, and low recyclability. Some of the genuine constraints of the regular technique, the investigation of the new shrewd materials and strategies have proceeded. The material business worried about modern uses has been accepting advantages from the improvement of new properties on strands [18]. The significance in a wide scope of uses, for example, improved erosion obstruction, straightforward and antireflective coatings, useful materials of exceptional wettability, hostile to freezing, against the haze and hostile to snow to radio wires and windows and bio-roused oceanic materials and gadgets [13, 19], and so forth. With the advancement of materials advertise in recreation and open-air donning, the requirements for water-repellent textures have drastically expanded. As a kind of delicate, agreeable, warm, biodegradable, and easy material, cotton textures have been chosen to be the chief attire textures. In any case, the cotton textures can be handily wetted and recolored by fluids because of the plenteous hydroxyl bunches on its surface [20].
In this manner, various principal looks into and down-to-earth applications have been given to change the cotton wettability. Xu et al. [21] fabricated the super-hydrophobic surface on cotton textures by joining the surface harshness made by silica nanoparticles and zinc oxide Nano-rods and low surface vitality adjusted by DTMS. Zhang et al. [22] depicted an economical, effortless, and low-temperature course for the hydrophobic brocades by develo** c-pivot arranged ZnO Nanorods on their surface. Cotton textures joined by nonafluorohexyl-1-acrylate under concurrent radiation-initiated unite polymerization, which indicated stable hydrophobicity, had been integrated by Deng et al. [23]. Bae et al. [20] arranged hydrophobic cotton textures by the blend of the silica nanoparticles and a financially savvy water-repellent operator. Zhou et al. [24] built up a powerful and strong hydrophobic cotton texture for oil–water division. Wang and collaborators made a thermoplastic polyurethane tangle with dot on-string morphology by electro-spinning, which can isolate oil and water blends after additionally treated by hydrophobic Nano-silica [25]. They demonstrated that the functionalized materials with the switchable superoleophilicity and superoleophobicity can be utilized in exceptionally controllable oil/water partition. Xue et al. [26] had been effectively arranged super-hydrophobic cotton texture by sol–gel covering of TiO2, which brought about great UV-protecting property. Zhai et al. [27] has announced an ecological neighborly sans fluorine covering technique to develop hearty superhydrophobic textures by dunking in streamlined Ormosil arrangement and along these lines balanced covering with PDMS. Albeit numerous materials have been utilized for oil and water repellency, there are still a lot of difficulties to survive, for example, significant expense, poor recyclability, etc. Novel materials that are financially savvy and simple to-get-ready are still profoundly sought after for proficient oil and water repellency.
Comparing with the above literature for oil and water repellency, HDTMS used for surface coatings on cotton fabric with the help of other chemicals such as methyltrimethoxysilane [28], trimethylated silica (TMS) [29], C-6 perfluorinated acrylic copolymer and silica nanoparticles [9], Silica hydrosol [30], zinc oxide [31], and so on. The above chemical treatments are responsible for hydrophobicity of fabric surface. However, some of the chemicals are harmful during the preparation process and others are expensive in use. On account of above circumstances, a simple chemical modification method using hexadecyltrimethoxysilane (HDTMS) with ethanol which is a better method in comparison with other methods since it is an inexpensive, simple method and offers an easy adjustment of chemical composition required for a surface to show hydrophobic behaviors.
Despite the massive research on the hydrophobicity of cotton fabric, there have been a few studies about nonwoven cotton with a new chemical. In this study, we use hexadecytrimethoxysilane (HDTMS) as a new chemical that functionalized the nonwoven cotton to increase hydrophobicity. This work is especially for investigating new functional textile materials in the field of hydrophobicity for oil and water repellency.
2 Experimental details
2.1 Materials
100% cotton nonwoven fabric was used in this research. The fabric specification was 80 GSM, plain pattern structure, grey color, 0.702 mm thickness and cotton fiber. The 100% cotton fiber contains 85% cellulose, 4% pectin, 5% water, 1.5% proteins and others materials. The size of the fabric used in this research was 4 cm × 4 cm. The nonwoven fabrics were purchased from Shanghai **ao-tong Fabrics Company limited, Shanghai, China. The main chemical Hexadecyltrimethoxysilane (HDTMS) was purchased from the Shanghai chemical market, China. The HDTMS molecule contains a total of 65 atoms (s). There are 42 Hydrogen atom(s), 19 Carbon atom(s), 3 Oxygen atom(s), and 1 silicon atom. The chemical formula of HDTMS can therefore be written as C19H42O3Si. The other chemical ethanol was purchased from Shanghai Jiaying Chemical Company, China. Distilled water was used in the manufacturing process. The technical specification of different chemicals used in this research is shown in Table 1.
2.2 Methodology
The Chemical modification of hydrophobic cotton nonwoven fabric was conducted by the one-step method through chemical treatment with solutions of Hexadecyltrimethoxysilane (HDTMS). The endurance of the hydrophobic properties of fabrics was defined by the measurement of the WCA on the surface of the fabric after the modification. In a round-bottom flask equipped with a reflux condenser and a magnetic stirrer. A solution of Hexadecyltrimethoxysilane (HDTMS), ethanol, and nonwoven cotton was placed on the flax and then stirred the solution. We used a different amount of HDTMS and ethanol to observe the best hydrophobic surface. Also, there was a different time and stirring velocity to maintain the hydrophobic surface on the cotton nonwoven. Stirring was conducted for 5 h at 60 °C temperature at 10 stirring velocities. After completing stirring, the sample was placed at room temperature for 24 h to be dried. The Schematic illustration of the preparation of hydrophobic surfaces is shown in Fig. 1. In Fig. 1, it also represents the hydrophobic chain of HDTMS on cotton fabric hinder the water and oil penetrate into the fiber. In Fig. 2, the chemical application processes for preparation of hydrophobic surfaces on nonwoven cotton fabrics are illustrated.
2.3 Characterization
2.3.1 Scanning electron microscope (SEM) analysis
The surface morphology of the treated nonwoven cotton fabric was studied using scanning electron microscope (SEM) images. The images were captured using JSM-7800F, JEOL, Japan with an accelerating voltage of 5.0 kV.
2.3.2 Fourier transform infrared spectroscopy (FTIR) analysis
To identify the bond arrangement in nonwoven cotton fabric with hexadecyltrimethoxysilane (HDTMS) using FTIR analysis. The analysis was carried out using Bruker spectrometer, Model: Tensor-27, with a golden gate single-reflection diamond ATR accessory. The infrared spectrums were recorded from the range of 4000 to 400 cm−1.
2.3.3 Determination of hydrophobic properties with water contact angle (WCA) analysis
The water contact angles were measured using an automatic video contact-angle testing apparatus, the Krüss model DSA 100 Expert. It measures the wettability of liquids on solid surfaces by the Young equation. The measurement procedures of WCA are shown in figure S1. A particular solid, liquid, and vapor system at a given heat and compression has a single equilibrium contact angle [32]. Though, in exercise, dynamic phenomena of contact angle lag are often perceived, going from a forward (maximum) contact angle to a backward (minimum) contact angle. Balanced contracts are within these values and can be considered from them. The balance contact angle reproduces the comparative strength of the interaction of liquid, solid, and vapor molecules. A 10-µl volume of water was applied to the treated cotton fabrics, and the contact angle was determined from the video camera images of the drop in the course of its formation. Each measurement is an average of five drops. The measurement by the video camera is shown in the supplementary video Movie-1.
2.3.4 Water repellency analysis
2.3.4.1 Spray test
The specimens of the size (180.0 × 180.0 mm) were cut from the test fabric. And the test specimens were conditioned at 65% relative humidity and 21 °C for a minimum of 4 h before testing. Then, the test specimen was fastened securely in the 152.4 mm diameter hoop. For this reason, the face of the fabric specimen was exposed to the water spray. Then, the pour 250 mL of distilled water at 27 °C into the funnel of the tester and allowed it to spray onto the test specimen for 25–30 s. Then, the changes in the specimen and the sticking or wetting of the specimen face were assessed, according to AATCC Test Method 22–2014 that was technically equivalent to ISO 4920.
2.3.4.2 Rain test
A minimum of three specimens of 20 × 20 cm was cut from the test fabric. Then, the fabric samples and the blotting paper were conditioned in an atmosphere of 65% RH and 21ºC for at least 4 h before testing. Then test specimen was backed by a (15.2 × 15.2 cm) standard paper blotter weighed to the nearest 0.1 g was clamped in the specimen holder. A horizontal water spray at 27 °C was directed against the specimen and was allowed to continue for 5 min. At the end of the spray period, the blotter was carefully separated and quickly reweighed to the nearest 0.1 g. Then, the changes in water penetration for each specimen calculated through the method of AATCC 35–2013 that was technically equivalent to ISO 22,958.
2.3.5 Oil repellency analysis
2.3.5.1 Hydrocarbon resistance test
Specimen size used to sufficient to allow for the complete range of test liquids to be evaluated, but shall be no smaller than 20 × 20 cm and no larger than 20 × 40 cm. Then, the test specimen was conditioned at 65% relative humidity and 21 °C for a minimum of 4 h before testing. Then, the test specimen was flat on the white textile blotting paper on a smooth, horizontal surface. After that, the AATCC Oil Test Grade Liquid carefully placed small drops approximately 5 mm in diameter on the test specimen in five locations along the filling direction. Observed the drops for 30 s, from approximately a 45° angle. Then, the changes in water penetration or wetting for each specimen were calculated through the method of AATCC 118–2013 that was technically equivalent to ISO 14,419.
3 Results and discussion
3.1 Mechanism of hexadecyltrimethoxysilane (HDTMS) on nonwoven cotton fabric
The general concept for the coated hexadecyltrimethoxysilane (HDTMS) on nonwoven cotton fabric is the cross-linking between cellulose and HDTMS. The schematic illustration is shown in Fig. 3. When ethanol is added to the nonwoven surface, then more –OH groups are exposed from the cellulose. The hexadecyltrimethoxysilane (HDTMS) are easily cross-linking with the nonwoven cotton fabric and make the surface coating. The HDTMS have a long-chain chemical structure. This long chain are also responsible for creating a long-chain structure with nonwoven cotton fabric. In the presence of ethanol, The HDTMS shows its role for making a long-chain cross-linking with nonwoven cotton fabric which ensure the functionalization of hydrophobic surfaces on nonwoven cotton.
3.2 SEM analysis
The morphological changes of the nonwoven cotton fabric caused by HDTMS under optimized condition were investigated by SEM and EDX Spectrum. It also ensures the particles were present on the surface of nonwoven cotton fabric in Fig. 4. Fig. 4a, b is the control nonwoven cotton fabric, and Fig. 4c is the HDTMS-treated nonwoven cotton fabric. It is visible that the HDTMS particles are on the treated nonwoven cotton fabric. The morphographs also demonstrated that HDTMS makes bonded on the surface of nonwoven cotton fabric. In Fig. 4d, EDX spectrum of treated nonwoven cotton fabric represents that the molecules from HDTMS are also present in the surface of nonwoven cotton. It gives the evidence that HDTMS are bonded with nonwoven cotton fabric.
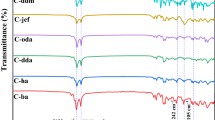
3.3 FTIR spectra analysis
To confirm the durable attachment of modifiers to the fabric surface, FTIR analysis has been performed (Figure S2 and S3). Figure 5a, b are the spectra of the control and treated fabrics subjected to chemical modification with hexadecyltrimethoxysilane. The transmittance is plotted in the curve which are obtained from the ATR accessory. The obtained transmittance from Bruker spectrophotometer is represented in Figure S2 and S3. In the visible spectra are differences resulting from the attachment of the organosilicon compound to cellulose hydroxyl groups. The band at 3329 cm−1, which is characteristic of the free OH groups present on the fiber surface, is slightly smaller, which indicates bonding between the fiber and alkoxysilyl groups. Moreover, the spectra of the treated samples contain bands at about 552 and 1087 cm−1, originating from Si–O–Si symmetric stretching vibrations and Si–O–C stretching vibration shoulder, respectively.
In the spectra, differences are visible, resulting from the attachment of organosilicon compounds to the cellulose hydroxyl groups. The band at 3329 cm−1is slightly smaller, which indicates bonding between the fiber and alkoxysilyl groups. Moreover, the spectra of the modified samples contain bands at about 552 and 1087 cm−1, the origin of which was mentioned above.
3.4 Water contact angle (WCA) analysis
Samples of fabrics were modified in a one-step process. The modifications were carried out at 50 °C, 60 °C and, 70 °C. For all the samples, measurements of the contact angle were conducted directly after the modification. At the initial stage of the study, the effect of the modification process duration on hydrophobic properties was determined. To this end, the modification with hexadecyltrimethoxysilane (HDTMS) was conducted for 50, 60, and 70 °C, and WCA on such a modified surface was measured (Table 2). Table 2 represents random experimental part for selection of ideal conditions for carry out the research. Here, experiment-08 indicates the overall ideal conditions with maximum contact angle. Others experiments are classified according to the change of different parameters.
3.4.1 Influence of different amount of HDTMS on nonwoven cotton
There is a great influence on the amount of HDTMS when all the materials and parameters are the same. In the first experiment, we use 3 ml HDTMS and 50 ml ethanol at 60 °C temperature at a stirring velocity of 10 for 10 h. Then, we measure the contact angle of the sample. We noticed that the contact angle of the sample. But in the next experiment, we verify the amount of HDTMS to know about the impact of the amount of HDTMS on nonwoven (Table 3). Later we use 2 ml, 4 ml, 5 ml, 6 ml and get different types of contact angles for that as shown in Figs. 6 and 7. In Fig. 7, it is shown that the contact angles are increasing with the increase in HDTMS added. The curve illustrates that the more of HDTMS, the higher of contact angles. Mainly, HDTMS make cross-linking of hydrophobic chain on cotton surface. So, the amount of HDTMS on cotton fabrics is significant for functionalization of hydrophobic nonwoven cotton. If we increase the further HDTMS, then the contact angles will be increased. Therefore, to minimize the cost and amount of HDTMS, other parameters are considered for optimization.
3.4.2 Influence of temperature on nonwoven cotton
There has a significant impact of temperature on nonwoven cotton while in hydrophobicity. We were seen that if all the parameters are the same but due to changing the temperature the result shows that there has a great chance of contact angle. While we test for the influence of temperature, we take 2 ml HDTMS and 40 ml ethanol and we change the temperature by 40 °C, 50 °C, 60 °C, and 70 °C (Table 4). We get the impact of different temperatures by measuring the contact angle for different temperatures as shown in Figs. 8 and 9. With the increase in temperature, the contact angle are also increased upto 60 °C. Because, with the increase in temperature, the more –OH groups are exposed in the solution and the HDTMS are make more cross-linking with non-woven cotton fabric. After that, the contact angles are decreased. So, the optimized temperature is selected as 60 °C. There is a reason for decreasing of contact angle after 60 °C temperature. The boiling point of ethanol is 78.24 °C. Hence, the increased temperature hinders the exposed of more –OH groups in the solution. Another point is that, the higher temperature can degrade the properties of nonwoven cotton fabric. So, the maximum contact angle of 123.7° was observed at 60 °C.
3.4.3 Influence of stirring time on nonwoven cotton
There is a significant impact of stirring time on nonwoven cotton while in hydrophobicity. We were seen that if all the parameters are the same but due to changing the stirring time the result shows that there is a change of contact angle. While we test for the influence of stirring time, we take 2 ml HDTMS and 40 ml ethanol at 60 °C temperature and we change the stirring time by 2 h, 3 h, 4 h 5 h, and 10 h (Table 5). We get the impact of different temperatures by measuring the contact angle for a different stirring time as shown in Figs. 10 and 11. The maximum contact angle of 127.4° was observed at 3 h. With the increase in time duration, the contact angles are relatively decreased. In the stirring bath, if the time is more, then the nonwoven cotton are placed in the reflux in a long time. After a certain time, the nonwoven degraded its properties, which causes the lower of contact angles. Basically, after 4 h, contact angles are decreased.
3.4.4 Influence of different amount of ethanol on non-woven cotton
There is little influence on the amount of ethanol when all the materials and parameters are the same. In the first experiment, we use 3 ml HDTMS and 50 ml ethanol at 60 °C temperature at a stirring velocity of 10 for 10 h. Then, we measure the contact angle of the sample. We noticed that the contact angle of the sample (Table 6). But in the next experiment, we verify the amount of ethanol to know about the impact of the amount of ethanol on nonwoven. Later, we use 40 ml, 60 ml, 70 ml and get different types of contact angles for that as shown in Figs. 12 and 13. The observed contact angles were almost similar.
3.4.5 Measurement of water contact angle by time
For this measurement, we were taken an optimized sample with a 127.4° contact angle. The preparation was using 2 ml HDTMS, 40 ml ethanol, 60 °C temperature at 3 h. We observe the contact angle every 5 min to know the change of contact angle. Here, we notice that the contact angle of the sample is changing by time (Fig. 14). We noticed that at 0 min the contact angle of the sample was 127.4° and at 115 min 120°.
3.4.6 Optimized HDTMS-treated nonwoven cotton fabric
The prepared HDTMS-treated cotton fabric was subjected to WCA analysis. By changing different parameters, we observed the different WCA. From among all the samples, we have taken three optimized samples for further oil and water repellency measurement. The listed are in Table 7.
3.5 Visual assessment
This is the visual test done by the drop of oil and water on the fabric surface to assess the oil and water resistance of nonwoven cotton fabric. Here, Fig. 15a is the control nonwoven cotton fabric and Fig. 15b is the HDTMS-treated nonwoven cotton fabric. In Fig. 15b, the drops of oil and water remain on the surface of the nonwoven fabric which ensures the treated fabric is hydrophobic. For better understanding of oil and water repellency by eye visualization, a color droplet test was done which is shown in Fig. 16. The image gives the clear result of oil and water repellency.
3.6 Water repellency analysis
3.6.1 Spray test
After 15 wash, the three optimized samples are spray tested for water repellency analysis. The results of water repellency spray test are presented in Fig. 17. Rating of the samples were done by using spray test rating chart. For the sample 1 and sample 2, AATCC value 100 rating indicates that the water drops completely repelled by the treated nonwoven fabric surface. For the sample 3, the value 90 indicates that the water drops slight random sticking or wetting of the fabric face. Both results of three samples are standard water repellency ratings [33, 34]. The spray tests are shown in the supplementary video Movie-2.
3.6.2 Rain test
The optimized HDTMS-treated nonwoven fabrics were subjected to rain test for water repellency measurement. The results are shown in Table 8. This method is by measuring the permeability of the fabric resistant to spray water to predict its resistance to the permeability of the rain. In this test process, a certain amount of water with certain-water pressure and under specified time through the standard required spray nozzle onto fabric. After the test is completed, check the water absorption of the contrary side of the fabric and how much weight of water is absorbed. Here, the test result shown that 0.2 gm of weight of water absorbed by the sample-1 and sample-3. For the sample-2, the absorbed water is 0.3 gm (Fig. 18). The rain test results indicate that the HDTMS-treated nonwoven cotton has satisfactory water repellency properties. The rain tests are shown in the supplementary video Movie-3.
3.7 Oil repellency analysis
3.7.1 Hydrocarbon resistance test
The step-by-step hydrocarbon resistance test for oil repellency is shown in Table 9. Here, the sample-1, AATCC rating is 6 and, in sample-2 and sample-3, the AATCC rating is 5 (Fig. 19). The above rating ensure the standard oil repellency properties developed on the surface of treated nonwoven cotton fabric [35].
4 Discussion
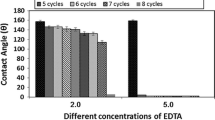
The three optimized samples are shown in the acceptable range of oil and water repellency properties as well as water contact angles (WCA). The overall results are shown by statistical analysis in Fig. 20. Here, the best results come from sample-1 and sample-2. For cost minimization and considering other properties, we select sample-2 for ideal candidate of oil and water repellency. After the different test of oil and water repellency, a microscopic images have taken for better understanding of the surface of nonwoven cotton fabric (Fig. 21). In Fig. 21a, b, the pores of nonwoven are not visible which means the HDTMS are coated on the surfaces remaining after different test. The HDTMS are more effective as hydrophobic agent than ethanol because HDTMS are long-chain chemical compounds. The long chain ensure the cross-linking between cellulosic cotton and HDTMS. The treated fabrics are also durable for a long time. After 20 washing cycles, there is a little difference of changes of contact angles (Table 10). This indicates that the treated fabric can be used for a long time. HDTMS treated with other chemicals such as silicone dioxide gives superhydrophobic properties of cotton fabric which are costly [36]. With comparing the other treatment on cotton fabric for oil and water repellency, our research carry out the best result with low cost and simple chemical modification methods. A comparison with our present study is represented in Table 11.
5 Conclusion
In this study, the nonwoven cotton fabric was treated with hexadecyltrimethoxysilane (HDTMS) and ethanol. Here, a chemical modification was done with HDTMS on a nonwoven cotton surface. The study has shown that hexadecyltrimethoxysilane (HDTMS) is a very good hydrophobic agent. A very simple one-step method of fabric modification was developed. A little amount of HDTMS is required to make the fabric hydrophobic. The temperature was also in the control range. Though we used a different amount of HDTMS and ethanol, we get the best result from 2 ml HDTMS with 40 ml ethanol at 60 °C. The water contact angle (WCA) of optimized treated nonwoven cotton fabric was 126 ± 2°. The nonwoven cotton fabrics treated with HDTMS shown excellent oil and water repellency properties. So, the HDTMS-treated nonwoven cotton fabrics are very promising and can be considered as the potential candidate to meet the actual demand of oil and water-repellent fabric in the textile industry.
Data availability
All data generated or analyzed during this study are included in this study.
References
Dalawai SP et al (2020) Recent advances in durability of superhydrophobic self-cleaning technology: a critical review. Prog Org Coat 138:105381
Kwon J, Jung H, Jung H, Lee J (2020) Micro/nanostructured coating for cotton textiles that repel oil, water, and chemical warfare agents. Polymers 12(8):1826
Hosseini Ravandi SA, Valizadeh M (2011) Properties of fibers and fabrics that contribute to human comfort. In: Song G (ed) Improving comfort in clothing, Woodhead publishing series in textiles. Woodhead Publishing, Elsevier, pp 61–78. https://doi.org/10.1533/9780857090645.1.61.
Bashar MM, Khan MA (2013) An overview on surface modification of cotton fiber for apparel use. J Polym Environ 21(1):181–190
Tian X, Bai H, Zheng Y, Jiang L (2011) Bio-inspired heterostructured bead-on-string fibers that respond to environmental wetting. Adv Funct Mater 21(8):1398–1402
Park S, Kim J, Park CH (2015) Superhydrophobic textiles: review of theoretical definitions, fabrication and functional evaluation. J Eng Fibers Fabr 10(4):155892501501000420
Ahmad I, Kan C-W (2016) A review on development and applications of bio-inspired superhydrophobic textiles. Materials 9(11):892
Mohsin M, Carr C, Rigout M (2013) Novel one bath application of oil and water repellent finish with environment friendly cross-linker for cotton. Fibers Polym 14(5):724–728
Zahid M, Heredia-Guerrero JA, Athanassiou A, Bayer IS (2017) Robust water repellent treatment for woven cotton fabrics with eco-friendly polymers. Chem Eng J 319:321–332
Walther A, Timonen JV, Díez I, Laukkanen A, Ikkala O (2011) Multifunctional high-performance biofibers based on wet-extrusion of renewable native cellulose nanofibrils. Adv Mater 23(26):2924–2928
Murakami D, **nai H, Takahara A (2014) Wetting transition from the Cassie–Baxter state to the Wenzel state on textured polymer surfaces. Langmuir 30(8):2061–2067
Hejazi V, Moghadam AD, Rohatgi P, Nosonovsky M (2014) Beyond Wenzel and Cassie–Baxter: second-order effects on the wetting of rough surfaces. Langmuir 30(31):9423–9429
Yao X, Song Y, Jiang L (2011) Applications of bio-inspired special wettable surfaces. Adv Mater 23(6):719–734
Li X, Cao M, Shan H, Tezel FH, Li B (2019) Facile and scalable fabrication of superhydrophobic and superoleophilic PDMS-co-PMHS coating on porous substrates for highly effective oil/water separation. Chem Eng J 358:1101–1113
Albrecht W, Fuchs H, Kittelmann W (2006) Nonwoven fabrics: raw materials, manufacture, applications, characteristics, testing processes. Wiley, Hoboken
Doh SJ, Lee JY, Lim DY, Im JN (2013) Manufacturing and analyses of wet-laid nonwoven consisting of carboxymethyl cellulose fibers. Fibers Polym 14(12):2176–2184
Das D, Pradhan AK, Chattopadhyay R, Singh S (2012) Composite nonwovens. Text Prog 44(1):1–84
Ogunmokun FA, Liu Z, Wallach R (2020) The influence of surfactant-application method on the effectiveness of water-repellent soil remediation. Geoderma 362:114081
Li Y, Duan G, Cai W (2007) Controllable superhydrophobic and lipophobic properties of ordered pore indium oxide array films. J Colloid Interface Sci 314(2):615–620
Bae GY, Min BG, Jeong YG, Lee SC, Jang JH, Koo GH (2009) Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J Colloid Interface Sci 337(1):170–175
Xu L, Zhuang W, Xu B, Cai Z (2011) Fabrication of superhydrophobic cotton fabrics by silica hydrosol and hydrophobization. Appl Surf Sci 257(13):5491–5498
Zhang M, Wang S, Wang C, Li J (2012) A facile method to fabricate superhydrophobic cotton fabrics. Appl Surf Sci 261:561–566
Deng B et al (2010) Laundering durability of superhydrophobic cotton fabric. Adv Mater 22(48):5473–5477
Zhou X et al (2013) Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl Mater Interfaces 5(15):7208–7214
Zhang L, Zhang Z, Wang P (2012) Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: toward controllable oil/water separation. NPG Asia Mater 4(2):e8–e8
Xue C, Jia S, Zhang J (2009) Preparation of fluoro-containing superhydrophobic cotton fabric with TiO2 sol–gel method. Dye Finish 35:1–4
Zhai L, Cebeci FC, Cohen RE, Rubner MF (2004) Stable superhydrophobic coatings from polyelectrolyte multilayers. Nano Lett 4(7):1349–1353
Ou J, Wang F, Li W, Yan M, Amirfazli A (2020) Methyltrimethoxysilane as a multipurpose chemical for durable superhydrophobic cotton fabric. Prog Org Coat 146:105700
Moiz A, Vijayan A, Padhye R, Wang X (2016) Chemical and water protective surface on cotton fabric by pad-knife-pad coating of WPU-PDMS-TMS. Cellulose 23(5):3377–3388
Zhao J, Yu J, Yan X, Jiangsu DT (2017) Research on water-repellent finishing for worsted fabrics by hydrosol method. J Jiangsu Coll Eng Technol 3:81–92
Xue C-H, Yin W, Zhang P, Zhang J, Ji P-T, Jia S-T (2013) UV-durable superhydrophobic textiles with UV-shielding properties by introduction of ZnO/SiO2 core/shell nanorods on PET fibers and hydrophobization. Colloids Surf, A 427:7–12
Good RJ (1992) Contact angle, wetting, and adhesion: a critical review. J Adhes Sci Technol 6(12):1269–1302
Kale KH, Palaskar S (2011) Atmospheric pressure plasma polymerization of hexamethyldisiloxane for imparting water repellency to cotton fabric. Text Res J 81(6):608–620
Maqsood M, Nawab Y, Hamdani STA, Shaker K, Umair M, Ashraf W (2016) Modeling the effect of weave structure and fabric thread density on the barrier effectiveness of woven surgical gowns. J Text Inst 107(7):873–878
Mohsin M, Farooq A, Abbas N, Noreen U, Sarwar N, Khan A (2016) Environment friendly finishing for the development of oil and water repellent cotton fabric. J Nat Fibers 13(3):261–267
Xu L, Wang L, Shen Y, Ding Y, Cai Z (2015) Preparation of hexadecyltrimethoxysilane-modified silica nanocomposite hydrosol and superhydrophobic cotton coating. Fibers Polym 16(5):1082–1091
Pipatchanchai T, Srikulkit K (2007) Hydrophobicity modification of woven cotton fabric by hydrophobic fumed silica coating. J Sol Gel Sci Technol 44(2):119–123
Ferrero F, Periolatto M, Sangermano M, Songia MB (2008) Water-repellent finishing of cotton fabrics by ultraviolet curing. J Appl Polym Sci 107(2):810–818
Ivanova N, Zaretskaya A (2010) Simple treatment of cotton textile to impart high water repellent properties. Appl Surf Sci 257(5):1800–1803
Acknowledgements
This work was jointly supported by the Chinese Government CSC Scholarship Program (CSC Number: 2019SLJ019821 and 2018SLJ020416) and State Key Laboratory of New Textile Materials and Advanced Processing Technologies, Wuhan Textile University, Wuhan 430200, People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 3978 KB)
Supplementary file3 (MP4 13110 KB)
Supplementary file4 (MP4 12196 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saha, P.K., Mia, R., Zhou, Y. et al. Functionalization of hydrophobic nonwoven cotton fabric for oil and water repellency. SN Appl. Sci. 3, 586 (2021). https://doi.org/10.1007/s42452-021-04582-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04582-9