Abstract
The most paramount challenge faced globally by the physicians is to treat the infections caused by nosocomial multi-drug resistant microorganisms. These bacteria are endowed with unique efflux pumps, which are substrate specific and a major defense mechanism for the bacteria. In fact, among various etiologies, the expulsion of antibiotics through efflux pump/s stands alone as an important factor for such resistance which these bacteria develop with time. To unravel this problem, several efflux pump inhibitors (EPI), which augment the efficacy of antibiotics, have been proposed to be administered along with antimicrobial agents nevertheless, none of them have entered in clinical use yet. In this review, we provide an overview of some of the nanoparticles postulated against multidrug resistance efflux pumps, and the inhibition strategies of EPIs action of some of the nanoparticles was searched along with EPIs which have been postulated for blocking the efflux pumps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Efflux pumps play a significant role of drug detoxification in various type of bacteria [1]. Evolution of efflux pumps is still in the debate, with few researchers believe that these pumps emerged due to the bacterial stress while others retain faith in their ancestral origin [2, 3]. Nevertheless, the theme preserves its uniqueness in that they are present in antibiotic susceptible as well as resistant bacteria and may be specific or act on several substrates [4]. Their enhanced expression may even give rise to multi-drug resistance. Resistance towards several antibiotics is a medical apprehension particularly when the field of vision is nosocomial infections [4]. Efflux pumps are grouped in five families comprising: major facilitator superfamily (MFS), ATP-binding cassette (ABC), small multidrug resistance (SMR), resistance-nodulation-division (RND), and the multidrug and toxic compound extrusion (MATE). Depending on their group families they are single-component transporters or multiple-component systems [5]. The single-component transporters containing inner membrane transporter such as MATE and SMR type efflux pumps and the multiple-component systems containing inner and outer membrane channel and a membrane fusion protein, such as the RND type efflux pumps that couple to proton motive force. Their tripartite arrangement allows the bacteria to direct extrusion of toxic drugs from cytosol or periplasmic space to the outside of bacterial by using the proton-gradient as an energy source [2, 6]. The expression of RND pumps is often regulated by local regulators (encoded upstream of the operon) and global regulators (like bile salt and fatty acid) [6].
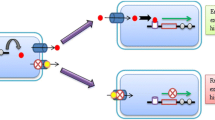
RND and MFS families of efflux proteins have an imperative role in the intrinsic drug resistance among gram-negative and gram-positive bacteria [5,6,7,8]. RND, ABC and MFS family efflux pumps can transport drugs by a three-step rotating mechanism in gram negative bacteria. Structural and functional of efflux pumps was shown in Fig. 1. At first potential substrates have access to enter when the entrance is kept open and the substrates move through the channel made by membrane fusion protein [9]. Then, in the efflux state, the entrance is locked and the exit is unlocked because of the conformational changes of the efflux pump that coupled to catalysis in F1Fo-ATPase or the proton motive force [2, 9]. After the extrusion of the substrate, the efflux pump turns back to the Access state for the other substrate [9]. RND system can efflux aminoglycosides, β-lactams, chloramphenicol, erythromycin, and tetracycline and ethidium bromide and reduce susceptibility to fluoroquinolones [10]. MFS system can efflux tetracycline, minocycline and chloramphenicol. MATE family can extract norfloxacin, ofloxacin, ciprofloxacin, gentamicin, daunorubicin, doxorubicin, rhodamine 6G and ethidium bromide [10]. RND system is a part of the bacterial protection system against both reactive oxygen species (ROS) and the host defense mechanisms. Adjunct to this, they play an effective role in the virulence of bacterial pathogens including; Colonization, twitching motility, toxin and biofilm production [6, 11]. Hence, the alternative for the future is the identification of molecules that inhibit efflux pumps so as to reduce drug resistance and bacterial pathogenesis [6, 11].
Efflux pump inhibitor (EPI) are described as weapons of anti-resistance by acting as an adjuvant of antibiotics, inducing an effect on the efflux pumps but retaining the activity of an antibiotic [5, 7] Many compounds have been tested and suggested for their efflux pump inhibition ability including some analogues for antibiotic substrates and other chemical compounds, but few of them take into consideration the structure–activity relationship and the spectrum of the activity [12]. They block the efflux family pumps as combinational therapy [13]. Efflux pump inhibitors (EPI), which may be natural or chemically synthesized compounds are usually considered only for their properties as antibiotic adjuvants, while their anti-virulence potential is seldom taken into account [12, 14]. Interestingly, EPIs are also used as an indicator to confirm the presence of active efflux pumps, for example in Pseudomonas aeruginosa [15].
EPI activity of phenylalanine arginyl b-naphthylamide (PAbN) is evidenced to counteract over expression of MexAB, MexCD, and MexEF efflux pumps in P. aeruginosa and other efflux pumps in gram-negative bacteria including Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae and Salmonella enterica [13, 16,17,18,19]. Phenothiazines and their derivatives such as chlorpromazine or thioridazine are other classes of EPIs substantiated to reduce antibiotic resistance in Burkholderia pseudomallei and S. enterica [20, 21]. Amongst EPIs, heterocyclic derivative of benzochromene (BC9) has been identified as a specific NorA efflux pump (MFS family) inhibitor thereby mitigating ciprofloxacin resistance. Concomitant use of antibacterial agents with EPIs has been suggested to reinvigorate antimicrobial agent as well as provide a synergistic effect and assist to combat with over-expression of efflux pumps in the resistant bacteria. However, none of efflux pump inhibitors have entered a clinical usage yet [16, 19]. Verapamil, an efflux inhibitor, has been profoundly found to decrease the MICs of bedaquiline and clofazimine in M. tuberculosis by 8- to 16-fold. Since the efflux pump mediated resistance is a significant antibiotic resilient mechanism in these bacteria, verapamil can be opted as an adjunctive chemotherapeutic agent for M. tuberculosis [22]. Another strategy revised for decreasing efflux pump activity is the disruption of the bacterial proton-motive force by using 2,4-dinitrophenol (DNP) and carbonyl cyanide m-chlorophenylhydrazone (CCCP) [23]. Similar to other EPIs, quinazoline has been found to possess an excellent activity to reduce the MIC of nalidixic acid, ciprofloxacin and chloramphenicol in gram-negative bacteria and to decrease overexpressed efflux pump AcrB, MexB and NorA activity in MDR strains [13, 19, 24]. The blocking activities of arylpiperazines compounds such as NMP on RND-type efflux pumps are modulated by the spatial structure of these compounds [13, 25]. NorA-(as a MFS family) and MepA-efflux pumps (as a MATE family) are shown to be inhibited by paroxetine which is one of the phenylpiperidine selective serotonin reuptake inhibitors (PSSRI) [26]. Liposome is another promising EPI agent. Fusion of liposome and an antibiotic execute the release of antibiotic into the bacterial cell forthwith [27].
Ethanol as a crude plant extract from Mentha avensis and other extracts from Turnera ulmifolia are some of the examples to take over the antibiotic resistance in clinical isolates of E. coli [28, 29]. Lemongrass oil (Cymbopogon citratus) has been shown to possess synergistic effect when used in conjunction with kanamycin and streptomycin on Salmonella typhimurium. Lemongrass oil and 50-methoxyhydnocarpin produced by barberry plants has been shown to block NorA pump in Staphylococcus aureus [13, 30] However, the main element from these extracts which inhibit efflux pump has not yet been identified [13].
Richard Feynman first theorized nanotechnology and is renowned as a Nobel-prize winner in 1959 [31]. Aftermath of his achievements made science of nanotechnology to creep as a new strategy to overcome the snags of antibiotic resistance [32]. For enhancing the therapeutic effectiveness of antibiotics, these have been encapsulated with nanoparticles [33]. Nanoparticles within the range size between 1 and 100 nm and high surface-to-volume ratio have an antimicrobial effect on MDR pathogens, which has been described as change in the membrane permeability, inhibition of multidrug efflux pumps, and damage to the chromosome [33, 34]. They combat with bacteria by using multiple mechanisms, thus emergence of resistance to them is probably unlike [34, 35]. The main antimicrobial effects of metal nanoparticles are due to the release of ions and production of reactive oxygen species (ROS) [34] such as superoxide (O2·–) ascending from electron transport processes of bacteria that is modified to hydrogen peroxide (H2O2) by superoxide dismutases (SOD) [36]. H2O2 is reduced to reactive hydroxyl radicals by redox-active metals (Fenton chemistry) [37,38,39]. They can damage DNA, RNA, cell membrane, proteins and bacterial oxidative phosphorylation and finally causing bacterial death [40]. The released ions can cause structural damage by creating hole in the bacterial membrane and thereby decrease the membrane integrity. They also interact with H+ gradient of the membrane. So, all energy dependent mechanisms in bacterial membrane such as the ABC super family efflux pumps and the others are suppressed followed by lethality of the bacteria [34].
2 Silver
Silver gained antibacterial feature since the ancient Greeks and its nano-crystalline form has demonstrated an unsurpassed antibacterial spectrum with efficacy on 150 different bacterial pathogens [41,42,43]. For centuries silver based compounds have been applied in the treatment of burn and chronic wounds and also used as eye drops for the prevention of trachoma [43]. It is also used as a silver-impregnated polymer in catheters for preventing the bacterial biofilm growth and in topical creams for healing burn wounds [41,42,43]. The antimicrobial effect of silver compounds is well documented, however, the inhibitory mechanism of silver on microbial growth is only partially known. It is suggested that the antimicrobial effect of silver nanoparticles is a combination of several reactions; penetration of the cell membrane causing structural changes in its permeability that cause accumulate of nanoparticles into the cell. So generation of free radicals is happened by ROS activation leading to a porous cell membrane, deactivation of vital enzymes, destruction of microbial DNA and RNA and finally the cell death of pathogenic microorganisms [44]. Silver ions interacts with chemical group of polymers present in bacterial membrane, reducing their integrity [45,46,47]. These ions can block electron transfer mechanism of bacteria by interacting with thiol groups of cysteine residues of NADH: quinone oxidoreductase (NQR) and inhibit its activity, failing many energy dependent mechanisms in bacteria [41, 48]. Silver ions have also been demonstrated to interact with nucleic acid and prevent its replication or even promote production of reactive oxygen species (ROS) that damages proteins, DNA, RNA and lipids [41]. For as much as silver nanoparticles (AgNPs) can produce further ionic silver, their ability for damaging bacterial cell wall, cell membrane proteins, DNA, RNA and lipids are increased [49,50,51]. Xu et al. [52]. and Lee et al. [53] independently synthesized and characterized purified spherically shaped silver nanoparticles (Ag NPs), and used the size-dependent localized surface plasmon resonance (LSPR) spectra of single Ag NPs to determine their sizes and to probe the size-dependent transport kinetics of the ABC transporters in single living cells (Bacillus subtilis) and P.aeruginosa respectively in real time at nanometer resolution using dark-field optical microscopy and spectroscopy (DFOMS). They showed the smaller NPs reside longer inside the cells than larger NPs, suggesting size-dependent efflux kinetics of the membrane transporter [52, 53]. Similar research on size-dependent plasmonic spectra of single NPs to probe the size-dependent transport kinetics of MexAB-OprM (multidrug transporter) in P.aeruginosa in real-time at nanometer resolution later confirmed that the accumulation of intracellular NPs in wild-type (WT) cells was higher than in over-expressed MexAB-OprM, but less than ΔABM (deletion of MexAB-OprM) [54]. They further demonstrated that residual time of NPs inside the cells increased in the presence of efflux pump inhibitor, Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP) [54]. Other invetigators studied the effects of AgNPs in the presence of chloramphenicol and aztreonam in P. aeruginosa which comprised several types of expression of MexAB-OprM [49, 52, 55]. Overall, these researchers investigated the role of the MexA-MexB-OprM efflux pump in controlling of the function of aztreonam (AZT) and chloamphenicol, in two mutants, nalB-1 (a mutant that overexpresses MexAMexB-OprM) and ∆ABM (a mutant devoid of MexAMexB-OprM). In the absence of AZT, in wild type (WT) strain, very few nanoparticles accumulated in nalB-1 and ∆ABM. The number of the nanoparticles remains almost unchanged over time. In 3.13 µg/mL AZT, unlike WT, very few Ag nanoparticles were observed in nalB1, and the number of Ag nanoparticles in nalB-1 remains unchanged over time. In contrast, the number of Ag nanoparticles accumulated in ∆ABM increases proportionally with time. In 31.3 µg/mL AZT, the number of Ag nanoparticles in nalB-1 and ∆ABM increases with time rapidly as observed in WT. Taken together, the results suggest that MexA-MexB-OprM plays an important role in the accumulation of the nanoparticles in the cells. Observations in these three strains, suggest that MexAMexB-OprM appears to effectively extrude nanoparticles at low AZT concentrations (0–3.13 µg/mL), at which concentrations the cellular membrane is still intact and its permeability is low while, at higher AZT concentration (31.3 µg/mL), the cellular wall is destroyed. Thus, the extrusion pump is unable to overcome an overflow of substrates (AZT and nanoparticles), and the number of Ag nanoparticles accumulated in any of these three strains increases rapidly with time, at a rate of 6-8-fold higher than in the absence of AZT. Similar results were demonstrated when the number of Ag nanoparticles accumulated in all three strains increased with the chloramphenicol concentration and incubation time, suggesting that chloramphenicol increases membrane porosity and permeability. In addition, the results indicate that accumulation kinetics of intracellular nanoparticles is associated with the expression levels of MexAB-OprM. The mutant with the overexpression level of MexAB-OprM (nalB-1) accumulates the least number of intracellular Ag nanoparticles, whereas the mutant devoid of MexAB-OprM (∆ABM) accumulates the greatest number of Ag nanoparticles. This suggests that MexAB-OprM plays a critical role in controlling of accumulation of intracellular Ag nanoparticles. These results are shown at Fig. 1. In the absence of the antibiotics, a lot of small AgNPs were observed in the WT (wild-type bacteria) and ∆ABM (deletion of MexAB-OprM), but few of intra cellular AgNPs were found in nalB-1 (over expression of MexAB-OprM) [52]. The staying time of AgNPs were increased on 10 fold concentration of 25 μg/mL chloramphenicol in the WT and ∆ABM strains, however, they were not changed in the nalB strains. The highest staying times of AgNPs were found in 250 μg/mL concentration of chloramphenicol in nalB strains. These results suggest that the permeability of the cellular membrane can be raised by increasing the chloramphenicol concentration [52]. AgNPs were found very few in nalB-1 and ∆ABM, unlike WT, in the presence of 3.13 μg/mL aztreonam. In the presence of 31.3 μg/mL AZT, the number of AgNPs in nalB-1 and ∆ABM increased with time like as observed in WT [55]. These results suggest the efflux pumps do not work properly because the cellular wall is damaged in the high concentrations of AZT that bind with nano particles. [55]. Figure 1 displays these results distinctly. Overall, Nancy and her colleagues showed that MexAB-OprM plays an important function in the gathering of NPs inside or efflux out of cells. These effects of efflux pumps can be reduced when NPs and antibiotics are used simultaneously [52, 54, 55]. Li with his colleagues in year 2005 studied synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles and found that nanosilver surrounded by antimicrobial groups can cause more destructive permeability and may act on the respiration functions of bacterial cell membranes [56]. Parallel to this, other investigations demonstrated that silver nanoparticles in combination with penicillin G, amoxicillin, erythromycin, clindamycin and vancomycin had a significant synergistic effect on the growth inhibition of bacteria with a considerable decrease in requirement dosages and their toxicity [56, 57]. Similar studies were followed by other researchers who loaded silver nanoparticles with antibiotics and used them against S. aureus, Micrococcus spp., E. coli, Salmonella typhi luteus and P. aeruginosa and could show significant growth inhibition [50, 58]. Proteomic and TEM analysis demonstrated that AgNPs cause bacterial death because of membrane damages and collapse of the proton motive force [53, 59]. The antibacterial activity and mechanism of silver nanoparticles (Ag-NPs) on Staphylococcus aureus was studied in China and the results showed an increase in the concentration of silver nano-particles with time makes cell DNA to condense to a tension state, loss of their replicating abilities, leading to the release of the cellular contents into the surrounding environments. Further, the investigation found Ag-NPs could reduce the enzymatic activity of respiratory chain dehydrogenase and with the proteomic analysis showed that the expression of some proteins was changed in the treated bacterial cell with Ag-NPs, namely formate acetyltransferase, aerobic glycerol-3-phosphate dehydrogenase, ABC transporter ATP-binding protein and recombinase A protein [60]. Effect of copper (Cu) and Ag nanoparticles has been shown to impair respiration of E. coli cell membrane leading to the damage in the bacterial membrane and decrease in the efflux pump activity [61, 62]. Figure 2 shows antimicrobial activities of silver nanoparticles evidently.
Structural and functional of five families of efflux pumps was shown. Major facilitator superfamily (MFS), small multidrug resistance (SMR) and resistance-nodulation-division (RND) and the multidrug and toxic compound extrusion (MATE) and ATP-binding cassette (ABC) in gram negative and gram positive bacteria
3 Zinc oxide
Zinc oxide nanoparticles (ZnO–NPs) harbors the potentiality to disrupt the cell membrane, generate the reactive oxygen species (ROS) and interact with DNA, RNA, proteins and lipids [63]. In a search for the agents to inhibit or take over the antibiotic resistance, ZnO nanoparticles were found to have synergistic effects with ampicillin, gentamicin, oxacillin, cloxacillin, amoxicillin, cephalexin, cefotaxime, ceftazidime, vancomycin, streptomycin, erythromycin, clindamycin, erythromycin, clindamycin, and tetracycline [64] whereas, in an another investigation these nanoparticles were found to lower the antibacterial activity of amoxicillin, penicillin G, and nitrofurantoin in S. aureus while, enhancing the antibacterial activity of ciprofloxacin in the presence of ZnO nanoparticles in both S.aureus and E.coli test strains have been seen. They proposed ZnO nanoparticles interfere with electron transfer kinetics that is energy source of NorA efflux pump. ZnO nanoparticles blocks this efflux pump to overcome the resistance towards fluoroquinolones in S. aureus [21, 65]. They also suggested that ZnO nanoparticles can interacts with membrane Omf protein. Omf protein is responsible for the constraint in the penetration of quinolones to the cell membrane so that ciprofloxacin absorption increases in the cell [65]. Furthermore, it is suggested that the inhibition of the efflux pumps and disruption of the cell membrane is due to the synergistic effect between antibiotics and NPs. The combination of ZnO–NPs with antibiotics may form a complex that can disturb the cell membrane of A. baumannii and facilitate the uptake of antibacterial agents [63]. Another investigation showed that the susceptibility of A. baumannii increased after exposure to combination of ZnO–NPs and antibiotics. The study suggested that this phenomenon may be due to the enhancement of membrane permeability and blocking of efflux pumps in this bacteria [63]. Combinations of nanoparticles and the antibiotic are advocated to cope up the antibiotic resistance as the combinations are not only more active against pathogens but also decreases the toxic dosage of antibiotics and nanoparticles, when administered individually [66]. Figure 2 depicts the antimicrobial activity of zinc oxide nanoparticle.
4 Chitosan
Chitosan is a linear polysaccharide composed of randomly distributed β-(1 → 4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit) that is derived from chitin [67]. The polymer is biocompatible, non-toxic, eco-friendly, and a safe drug carrier. Encapsulation of the temporin B (TB) into chitosan nanoparticles (CS-NPs) have been shown to increase its antibacterial activity [68]. Chitosan has positive charge at acidic pH condition, therefore, it can damage the negatively charge cell wall of bacteria. In an investigation performed on mode of antimicrobial action of chitosan (polymeric beta-1,4-N-acetylglucosamine) on gram-negative bacteria it was observed that this nanoparticle rendered E. coli more sensitive to the inhibitory action of dyes and bile acids used in selective media and on the other hand, highly cationic mutants of S. typhimurium were found more resistant to chitosan than the parent strains. Electron microscopy showed chitosan to cause extensive cell surface alterations and covered the outer membrane with vesicular structures, thus the NP appeared to bind to the outer membrane, explaining the loss of the barrier function [69]. In addition, chitosan does bind to DNA and inhibit the transcription and translation in microbial cells [34]. In year 2009, chitosan along with sulfamethoxazole was shown to reduce the minimum inhibitory concentration (MIC) value of sulfamethoxazole by fivefold in P. aeruginosa harboring a highly expressive MexEF-OprN efflux pump [70]. This result demonstrated that chitosan in combination with antibiotics can change expression of efflux pumps, although this findings further need some molecular investigations. Antibacterial activity of chitosan nanoparticle has been shown at Table 1.
5 Nitric oxide
Nitric oxide nanoparticles (NO NPs) uses several mechanisms to act on the bacteria. One of them is the production of reactive nitrogen oxide (RNOS) that can interact with bacterial proteins and eventually damages them. RNOS can also effect bacterial DNA and inhibit DNA repair enzymes. It interacts with zinc metalloproteinase and block respiration of bacteria [71] that cause decrease in efflux pumps activity. Earlier studies showed that action of NO NPs in concentration of 1.25–5 mM was enough to demonstrate antibacterial activity against MDR E. faecalis, K. pneumoniae, E. coli, P. aeruginosa and MRSA (methicillin resistant S. aureus; an MDR strain that over expresses efflux pump) [35, 72]. NONPs do have broad spectrum significant antimicrobial activity acting against both gram-positive and negative bacteria and these nanoparticles releases nitric-oxide (NO) that can bind to proteins and lipids of plasma membrane and change their integrity. NO can easily be transferred to the cytoplasm where it can interact with DNA, RNA and enzymes too [73]. Nonetheless, molecular study is necessary to understand NO NPs interactions with efflux pump. Antibacterial activity of nitric oxide nanoparticle has been shown in Table 1.
6 Magnesium oxide
Magnesium oxide nanoparticles (MgO NPs), another metal nanoparticle which stimulate ROS formation leading to the damage in the bacterial cell, is postulated to have a reverse effect on the efflux pumps of bacteria [34]. The antibiotic activity of MgO NPs increases after halogen molecules such as, Cl2, Br2 and F2 are adsorbed to these nanoparticles. Magnesium chloride (MgCl2), magnesium bromide (MgBr2) and magnesium fluoride (MgF2) nanoparticles have been documented to have bactericidal and anti-biofilm activity against Bacillus subtilis and S. aureus [34, 72, 74]. Figure 2 displays the antimicrobial activity of magnesium nanoparticles.
7 Copper
Comparative to other nanoparticles, these nanoparticles are less exploited. Diverse mechanisms of actions have been elucidated for copper including; direct interaction and permeabilization of the bacterial cell membrane, production of free radicals, RNA degradation, DNA strand breakage and cross linking, disordering DNA helical structure, DNA mutations, oxidation, mutation or cleavage of proteins, and displacement of essential metals of proteins [61, 75,76,77,78,79,80,81]. Enterococcus faecalis, S. aureus and E. coli are documented to be more susceptible to copper nanoparticles than P. aeruginosa [82]. Interestingly, copper nanoparticles have been demonstrated to act at various concentrations on different bacteria. For instance, the efflux pumps of S. aureus were observed to be inhibited at 0.032 mM concentration whereas, the efflux pumps of the wild type P. aeruginosa and S. aureus were highly inhibited at 0.065 mM concentrations [61]. Copper nanoparticles have been demonstrated to lower the MIC of the ciprofloxacin by functioning as an efflux pump inhibitor in MRSA [61]. Nor A is a predominant efflux pump in S. aureus that contributes to MDR phenotypes [21, 83] and copper nanoparticles inhibits the activity of Nor A. The same research investigation showed copper nanoparticles to cause significant biofilm inhibition in both P. aeruginosa and S. aureus at lower concentration [61]. The reason of susceptibility of biofilms at lower concentrations is associated with multiple antibiotic response regulators (Mar R) which are copper sensors. In the lower concentration of copper, cysteine residues (cys80) of Mar R change to tetramerization by disulfide bonds, so the transcription of the biofilm is stopped [84]. Copper ions from nanoparticles might act as signaling molecules in S. aureus and change gene expression of exopolysaccharide molecules. Anti-biofilm agents when used in combination with a number of efflux pump inhibitors significantly reduce biofilm formation and could even abolish biofilms in RND and MFS pumps expressing strains [82, 85]. Totally, efflux inhibitory and antibacterial effects of copper are partly mediated by copper (II) ions and partly mediated by copper nanoparticles. Antimicrobial activity of copper nanoparticle has been shown in Fig. 2.
8 Iron
Iron nanoparticle have been used as hyperthermia agents [86, 87], as carriers for targeted drug delivery to treat several types of cancer [88,89,90], as contrast agents for magnetic resonance imaging (MRI) [89, 91] and as bactericidal agents [92,93,94,95]. The bactericidal mechanisms of iron nanoparticle is due to the production of intracellular oxidative, such as OH, Fe(IV), generated by the reaction with hydrogen peroxide or other species [96], oxidative stress generated by reactive oxygen species (ROS) [96, 97], disruption of cell membrane integrity [93]. Bactericidal effects of iron nanoparticle under anaerobic conditions are greater than under air-saturated conditions [94]. Expulsion of rifampicin and other anti TB drugs in M. smegmatis are made effective by iron oxide nanoparticle covered with polyacrylic acid [98]. ROS generated by iron nanoparticle is the reason of damage of DNA and proteins in bacteria and probably impairment of the efflux pumps. The potential advantages of iron nanoparticle compared with others are easy preparation, low cost and high activity. Antimicrobial activity of iron nanoparticle has been shown at Fig. 2.
9 Calcium
Use of dental composites are growing due to their improved performance and esthetics. Dental composites consist of a polymerizable resin matrix, reinforcing glass particle fillers, and silane coupling agents which have good aesthetic properties and strength, making them the most widely used materials for the restorations of anterior teeth [99, 100] but composites used in vivo show that they are limited to time and may lead to plaque and biofilm formation [100, 101]. Therefore, improving the longevity of composite restorations is needed. The composite restorations should incorporate bioactive agents to combat recurrent caries, microbial destruction and sustaining the load-bearing capability [102]. Various classes of antibacterial dental composites are used including, polymerase containing QAS (quaternary ammonium salts) [103, 104], silver (Ag) [102, 105], calcium phosphate (CaP) particles [106, 107]. The CaP composites releases supersaturating levels of phosphate (PO4) and calcium ions and rematerialized tooth lesions [106, 107]. It has been demonstrated that the combination composites of calcium phosphate nanoparticle, QAS nanoparticle and silver nanoparticle greatly reduced the metabolic activity, biofilm growth, lactic acid production and colony forming units (CFU) counts of S.mutans compared with two other commercial composites [108]. In another study, when calcium hydroxide was used as an intracranial dressing, its bactericidal effect was found associated with its high pH (12.5–12.8) [109]. When an investigation was performed to evaluate the role of efflux pumps in altering the susceptibility of Enterococcus faecalis biofilms to calcium hydroxide [Ca(OH)2], chitosan nanoparticles, and light-activated disinfection (LAD), it was observed that E. faecalis biofilms were found to persist even after 24-h treatment with different concentrations of Ca(OH)2. On the other hand, LAD completely inactivated 4-day-old E. faecalis biofilms. The addition of EPI improved the antibiofilm efficacy of Ca(OH)2 at lower concentrations (P < 0.001), but had no effect on higher concentrations [110]. In contrast to the above findings, other researchers did not accepts calcium hydroxide as an effective agent against E. faecalis in infected tooth models [111, 112].
Antibacterial effect of calcium hydroxide is associated with calcium ions. The binding sites of calcium ions on gram positive bacteria are phosphate and carboxylate groups on the cell surface [113, 114]. No study has yet investigated effect of calcium nanoparticles on efflux pumps.
The potential bactericidal effect of calcium hydroxide is associated with calcium ions that facilitate the diffusion of OH− ions into the cell wall. Binding of calcium ions to the anionic groups, neutralize the anionic charges and reduce the repulsion of anionic entities of the cell wall [110]. Thus, the bactericidal mechanism of calcium nanoparticles may probably be due to the calcium ions released from the disassociation of calcium nanoparticles.
10 Platinum
Platinum is known to inactivate bacteria by interacting with their proteins, DNA and enzymes restraining cell division and cell proliferation [115]. Colloidal platinum nanoparticles have been assessed for their ability to reduce the pulmonary and epidermal inflammations [116, 117]. As platinum oxide has less environmental pollution compared with other metals, thus the metal in the form of nanoparticle is considered a proper candidate to combat with environmental pathogens [118]. Platinum oxide stabilized with sucrose exhibited bactericidal activity against lactobacillus species and Pseudomonas stutzeri [118]. Another investigation demonstrated that platinum nanoparticles with the 1–3 nm size exhibited bactericidal properties against clinical pathogen P. aeruginosa while the 4–21 nm exhibited bacterio-compatible properties in the same P. aeruginosa [119]. The anti-efflux pump effects of platinum have not been evaluated and the most studies of bactericidal effects of platinum nanoparticles have focused on the combination of platinum with other bactericidal components.
11 Gold
Gold nanoparticles are superior due to their controllable size, easily modifiable with desired molecules, chemically stable, and being nontoxic to mammalian cells and animals [120,121,122,123]. Gold nanoparticles have served as a flexible platform for exploring several aspects of basic sciences such as by linking to single molecules, conjugation with DNA, oligosaccharides, proteins or small bio functional molecules, finding applications in biology and assisting in fundamental developments in materials and physical science [89, 124]. Gold nanoparticles are an ideal model system to multivalency and polymeric based nanoparticles because they are 103 times smaller than bacterium (4–5 nm in diameter). In addition, they maintain a contrast shape and size in solution [125]. These nanoparticles have been found to enhance enhanced in vitro bactericidal activities against vancomycin-resistant Enterococcus (VRE), probably acting as a rigid polyvalent inhibitor [125]. Moreover, influence of gold nanoparticles conjugated to N-acetyl lactosamine have been observed to reduce the binding of EPEC (enteropathogenic Escherichia coli) to the epithelial cells and thus their localization [126]. Gold nanoparticles have been found to penetrate the cell wall of Corynebacterium pseudo tuberculosis and accumulate as intracellular agglomerates [127]. In an another study it was found that pyrimidine-presented on gold nanoparticles execute their antibiotic actions via sequestration of calcium and magnesium ions to disrupt the membrane of bacterial cells, resulting in the permeation of cytoplasmic contents such as nucleic acids and also act via inhibition of protein synthesis and interaction with DNA by internalized nanoparticles [128]. In the other studies vancomycin-resistant Enterococci (VRE) were shown to be susceptible to gold nanoparticles coated with vancomycin [58, 125]. Ampicillin conjugated to gold nanoparticles was observed to be effective as broad-spectrum antibacterial agent acting against both gram positive and negative bacteria such as E. aerogenes, P. aeruginosa and MRSA while, gold nanoparticles uncapped with ampicillin had no activity against these bacteria [129]. Results of this study concluded that blockage of efflux pump and multivalent presentation of ampicillin are probably the causes of more effective action of ampicillin-capped gold nanoparticles compared with ampicillin alone. In another contemplation, antibacterial activities of AuNPs were seen on the E. coli concluding that these nanoparticles act by inhibiting tRNA binding to the subunit of ribosome, collapse of membrane potential and impeding ATPase activities [128]. Attention paid by Zhao et al. from China and later Khameneh et al. from Iran to develop antibacterial nanodrugs so as to unwrap the antibiotic resistance revealed that gold NPs in combination with ampicillin had an effective antibacterial activity against high ampicillin resistance bacteria [32, 130]. They speculated that inhibition of the efflux pumps by the ampicillin bound to the gold NPs are the fundamental reason of the antibacterial effects of these agents. Despite these advances in the scientific world, gold nanoparticles alone could not erect the standards [32]. In search for new strategies to enhance antibacterial activity of antibiotics, the combination effect of gold materials including trivalent gold ions (Au3+) and gold nanoparticles (Au NPs) with 14 different antibiotics was investigated against P. aeruginosa, Staphylococcus aureus and Escherichia coli clinical isolates by disk diffusion assay. They demonstrated that the susceptibility of resistant P. aeruginosa increased in the presence of Au3 + and methicillin, erythromycin, vancomycin, penicillin G, clindamycin and nalidixic acid, up to 147%. Parallel to this, similar results were observed when the same group of antibiotics were tested against S. aureus, E. coli clinical isolates and a different P. aeruginosa resistant strain in the presence of sub-inhibitory contents of Au3 + , where Au3 + increased the susceptibility of test strains to methicillin, erythromycin, vancomycin, penicillin G, clindamycin and nalidixic acid. Their finding suggested that using the combination of sub-inhibitory concentrations of Au3 + and methicillin, erythromycin, nalidixic acid or vancomycin may be a promising new strategy for the treatment of highly resistant P. aeruginosa infections. However, more laboratory and molecular tests are required to be done in order to determine the effects of Au3 + and AuNPs in combination with antibiotics on P. aeruginosa cell wall permeability and their efflux pumps [131]. Antibacterial activity of gold nanoparticle has been shown in the Table 1 (Fig. 3).
Ag NPs, ZnO NPs, MgO NPs, CuO NPs and Iron nanoparticles can damage DNA, mRNA, and peptide of bacteria by effecting on production of reactive oxygen species (ROS) (it was shown by black dotted arrows). In addition, Ag NPs can damage the cell membrane, DNA, mRNA, peptide production, electron transport chains and on the efflux pumps directly (it was shown by black continuous arrows). Iron nanoparticles can damage the cell membrane directly (was shown by orange continuous arrow) and have indirectly effects on DNA, transcription and translation phases. ZnO NPs can damage the cell membrane and efflux pumps directly (was shown by purple continuous arrows) and has an indirect effect on DNA, mRNA and translation phase (was shown by dotted arrows). CuO NPs can damage the cell membrane directly (was shown by continuous arrow) and can effect on the translation and transcription phases indirectly
12 Conclusion
Nanoparticles provide important antibacterial strategies to combat bacterial resistance. Recent studies mostly focused on in vitro effects of nanoparticles. Therefore, analysis of in vivo antibacterial activities and toxicity of nanoparticles may accelerate the realization of metal nanoparticles-enabled antibiotics. In addition, bactericidal mechanisms of metal nanoparticles have not been completely evaluated. In this review we concluded that some of the nanoparticles provide bactericidal effects and may have not anti-efflux pump effects such as platinum and calcium hydroxide whereas, some of them provide both bactericidal and anti-efflux pump effects such as chitosan, silver, gold, iron and copper. Their antibacterial activity and anti-efflux pumps effects increased after combination with antibiotics. Further molecular and proteomics study are required to realize effects of nanoparticles on different classes of efflux pumps in the resistant bacteria.
References
Cao Q et al (2018) Haemophilus parasuis CpxRA two-component system confers bacterial tolerance to environmental stresses and macrolide resistance. Microbiol Res 206:177–185
Piddock LJ (2006) Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19(2):382–402
Webber M, Piddock L (2003) The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51(1):9–11
Yoon E-J, Courvalin P, Grillot-Courvalin C (2013) RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrobial Agents Chemother 57(7):2989–2995
Li X-Z, Nikaido H (2004) Efflux-mediated drug resistance in bacteria. Drugs 64(2):159–204
Fernando D, Kumar A (2013) Resistance-nodulation-division multidrug efflux pumps in gram-negative bacteria: role in virulence. Antibiotics 2(1):163–181
Piddock LJV (2006) Multidrug-resistance efflux pumps? Not just for resistance. Nat Rev Microbiol 4:629
Schweizer HP (2003) Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet Mol Res 2(1):48–62
Murakami S et al (2004) Extramembrane central pore of multidrug exporter AcrB in Escherichia coli plays an important role in drug transport. J Biol Chem 279(5):3743–3748
Vila J, Martí S, Sanchez-Céspedes J (2007) Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother 59(6):1210–1215
Thota N et al (2008) Citral derived amides as potent bacterial NorA efflux pump inhibitors. Bioorg Med Chem 16(13):6535–6543
Askoura M et al (2011) Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J Med 6(1):5870
Bolla J-M et al (2011) Strategies for bypassing the membrane barrier in multidrug resistant gram-negative bacteria. FEBS Lett 585(11):1682–1690
Rampioni G et al (2017) Effect of efflux pump inhibition on Pseudomonas aeruginosa transcriptome and virulence. Sci Rep 7(1):11392
Kriengkauykiat J et al (2005) Use of an efflux pump inhibitor to determine the prevalence of efflux pump-mediated fluoroquinolone resistance and multidrug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 49(2):565–570
Zowalaty MEE et al (2015) Pseudomonas aeruginosa: arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol 10(10):1683–1706
Yoshida KI et al (2007) MexAB-OprM specific efflux pump inhibitors in Pseudomonas aeruginosa. Part 7: highly soluble and in vivo active quaternary ammonium analogue D13-9001, a potential preclinical candidate. Bioorg Med Chem 15(22):7087–7097
Opperman T, Nguyen S (2015) Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol 6:421
Handzlik J, Matys A, Kieć-Kononowicz K (2013) Recent advances in multi-drug resistance (MDR) efflux pump inhibitors of gram-positive bacteria S. aureus. Antibiotics 2(1):28–45
Bailey AM, Paulsen IT, Piddock LJV (2008) RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob Agents Chemother 52(10):3604–3611
Couto I et al (2008) Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J Antimicrob Chemother 62(3):504–513
Gupta S et al (2014) Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58(1):574–576
Zechini B, Versace I (2009) Inhibitors of multidrug resistant efflux systems in bacteria. Recent Pat Anti-Infect Drug Discov 4(1):37–50
Chevalier J et al (2010) Quinazoline derivatives are efficient chemosensitizers of antibiotic activity in Enterobacter aerogenes, Klebsiella pneumoniae and Pseudomonas aeruginosa resistant strains. Int J Antimicrob Agents 36(2):164–168
Bohnert JA, Kern WV (2005) Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps. Antimicrob Agents Chemother 49(2):849–852
German N, Kaatz GW, Kerns RJ (2008) Synthesis and evaluation of PSSRI-based inhibitors of Staphylococcus aureus multidrug efflux pumps. Bioorg Med Chem Lett 18(4):1368–1373
Porras-Gómez M, Vega-Baudrit J, Núñez-Corrales S (2012) Overview of multidrug-resistant Pseudomonas aeruginosa and novel therapeutic approaches. J Biomater Nanobiotechnol 3(04):519
Coutinho HDM et al (2008) Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 54(4):328–330
Coutinho HDM et al (2010) Increasing of the aminoglicosyde antibiotic activity against a multidrug-resistant E coli by Turnera ulmifolia L. and chlorpromazine. Biol Res Nurs 11(4):332–335
Stermitz FR et al (2000) Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci 97(4):1433–1437
Macdonald RL (2015) Nanoparticles and microparticles. Neurosurgery 62(CN_suppl_1):152–159
Zhao Y, Jiang X (2013) Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 5(18):8340–8350
Zhang L et al (2010) Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem 17(6):585–594
Pelgrift RY, Friedman AJ (2013) Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 65(13):1803–1815
Huh AJ, Kwon YJ (2011) “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Controlled Release 156(2):128–145
Dedon PC, Tannenbaum SR (2004) Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys 423(1):12–22
Bataineh H, Pestovsky O, Bakac A (2016) Electron transfer reactivity of the aqueous iron (IV)–oxo complex. Outer-sphere versus proton-coupled electron transfer. Inorg Chem 55(13):6719–6724
Imlay JA (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11(7):443
Imlay JA (2015) Diagnosing oxidative stress in bacteria: not as easy as you might think. Curr Opin Microbiol 24:124–131
Pelgrift RY, Friedman AJ (2013) Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 65(13–14):1803–1815
Mijnendonckx K et al (2013) Antimicrobial silver: uses, toxicity and potential for resistance. Biometals 26(4):609–621
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371
Maillard J-Y, Hartemann P (2013) Silver as an antimicrobial: facts and gaps in knowledge. Crit Rev Microbiol 39(4):373–383
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2(1):32
Feng QL et al (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Li W-R et al (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85(4):1115–1122
Yamanaka M, Hara K, Kudo J (2005) Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol 71(11):7589–7593
Fadeeva MS et al (2011) Cys377 residue in NqrF subunit confers Ag+ sensitivity of Na+ -translocating NADH: quinone oxidoreductase from Vibrio harveyi. Biochemistry (Moscow) 76(2):186–195
Wijnhoven SWP et al (2009) Nano-silver—a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3(2):109–138
Birla SS et al (2009) Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol 48(2):173–179
Banerjee M et al (2010) Heightened reactive oxygen species generation in the antimicrobial activity of a three component iodinated chitosan–silver nanoparticle composite. Langmuir 26(8):5901–5908
Xu X-HN et al (2004) Real-time probing of membrane transport in living microbial cells using single nanoparticle optics and living cell imaging. Biochemistry 43(32):10400–10413
Lee KJ et al (2010) Probing of multidrug ABC membrane transporters of single living cells using single plasmonic nanoparticle optical probes. Anal Bioanal Chem 397(8):3317–3328
Nallathamby PD et al (2010) Study of the multidrug membrane transporter of single living Pseudomonas aeruginosa cells using size-dependent plasmonic nanoparticle optical probes. Biochemistry 49(28):5942–5953
Kyriacou SV, Brownlow WJ, Xu X-HN (2004) Using nanoparticle optics assay for direct observation of the function of antimicrobial agents in single live bacterial cells. Biochemistry 43(1):140–147
Li P et al (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 16(9):1912
Shahverdi AR et al (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed Nanotechnol Biol Med 3(2):168–171
Mohammed Fayaz A et al (2011) Vancomycin bound biogenic gold nanoparticles: a different perspective for development of anti VRSA agents. Process Biochem 46(3):636–641
Lok C-N et al (2007) Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem 12(4):527–534
Li W-R et al (2011) Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 24(1):135–141
Christena LR et al (2015) Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv 5(17):12899–12909
Choi O et al (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res 42(12):3066–3074
Ghasemi F, Jalal R (2016) Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multidrug-resistant Acinetobacter baumannii. J Glob Antimicrobial Resistance 6:118–122
Thati V et al (2010) Nanostructured zinc oxide enhances the activity of antibiotics against Staphylococcus aureus. J Biosci Technol 1(2):64–69
Banoee M et al (2010) ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B Appl Biomater 93B(2):557–561
Allahverdiyev AM et al (2011) Co** with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti-Infective Ther 9(11):1035–1052
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Piras AM et al (2015) Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front Microbiol 6:372
Helander IM et al (2001) Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol 71(2):235–244
Tin S et al (2009) Activity of chitosans in combination with antibiotics in Pseudomonas aeruginosa. Int J Biol Sci 5(2):153–160
Schairer DO et al (2012) The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 3(3):271–279
Hajipour MJ et al (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30(10):499–511
Friedman A et al (2011) Susceptibility of gram-positive and-negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence 2(3):217–221
Blecher K, Nasir A, Friedman A (2011) The growing role of nanotechnology in combating infectious disease. Virulence 2(5):395–401
Borkow G, Gabbay J (2005) Copper as a biocidal tool. Curr Med Chem 12(18):2163–2175
Rong X et al (2008) Interaction of Pseudomonas putida with kaolinite and montmorillonite: a combination study by equilibrium adsorption, ITC, SEM and FTIR. Colloids Surf B 64(1):49–55
Guo T et al (2011) Adsorptive property of Cu2+-loaded montmorillonite clays for Escherichia coli K88 in vitro. J Environ Sci 23(11):1808–1815
Walker SG et al (1989) Physicochemical interaction of Escherichia coli cell envelopes and Bacillus subtilis cell walls with two clays and ability of the composite to immobilize heavy metals from solution. Appl Environ Microbiol 55(11):2976–2984
Ruparelia JP et al (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4(3):707–716
Usman MS et al (2013) Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed 8:4467–4479
Ramyadevi J et al (2012) Synthesis and antimicrobial activity of copper nanoparticles. Mater Lett 71:114–116
Bagchi B et al (2013) In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids Surf B 108:358–365
Ng EY, Trucksis M, Hooper DC (1994) Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother 38(6):1345–1355
Hao Z et al (2014) The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat Chem Biol 10(1):21–28
Kvist M, Hancock V, Klemm P (2008) Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol 74(23):7376–7382
Chan DC, Kirpotin DB, Bunn PA (1993) Synthesis and evaluation of colloidal magnetic iron oxides for the site-specific radiofrequency-induced hyperthermia of cancer. J Magn Magn Mater 122(1):374–378
Gonzales-Weimuller M, Zeisberger M, Krishnan KM (2009) Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J Magn Magn Mater 321(13):1947–1950
Chertok B et al (2008) Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 29(4):487–496
**ng R et al (2013) Hollow iron oxide nanoparticles as multidrug resistant drug delivery and imaging vehicles. Nano Res 6(1):1–9
Kievit FM et al (2011) Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J Controlled Release 152(1):76–83
Beets-Tan RGH, Van Engelshoven JMA, Greve JWM (1998) Hepatic adenoma and focal nodular hyperplasia: mr findings with superparamagnetic iron oxide-enhanced mri. Clin Imaging 22(3):211–215
Borcherding J et al (2014) Iron oxide nanoparticles induce Pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ Sci Nano 1(2):123–132
Kim JY et al (2010) Inactivation of Escherichia coli by nanoparticulate zerovalent iron and ferrous ion. Appl Environ Microbiol 76(22):7668–7670
Lee C et al (2008) Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ Sci Technol 42(13):4927–4933
Tran N et al (2010) Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomed 5:277–283
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Kohanski MA et al (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130(5):797–810
Padwal P, Bandyopadhyaya R, Mehra S (2014) Polyacrylic acid-coated iron oxide nanoparticles for targeting drug resistance in mycobacteria. Langmuir 30(50):15266–15276
Lim BS et al (2002) Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater 18(6):436–444
Drummond JL (2008) Degradation, fatigue, and failure of resin dental composite materials. J Dent Res 87(8):710–719
Beyth N, Domb AJ, Weiss EI (2007) An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent 35(3):201–206
Yoshida K, Tanagawa M, Atsuta M (1999) Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J Biomed Mater Res 47(4):516–522
Imazato S (2003) Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 19(6):449–457
Tezvergil-Mutluay A et al (2011) The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res 90(4):535–540
Tanagawa M et al (1999) Inhibitory effect of antibacterial resin composite against Streptococcus mutans. Caries Res 33(5):366–371
Dickens SH, Flaim GM, Takagi S (2003) Mechanical properties and biochemical activity of remineralizing resin-based Ca–PO4 cements. Dent Mater 19(6):558–566
Langhorst SE, O’Donnell JNR, Skrtic D (2009) In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mater 25(7):884–891
Cheng L et al (2012) Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater 28(5):561–572
Byström A, Claesson R, Sundqvist G (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Dent Traumatol 1(5):170–175
Upadya M, Shrestha A, Kishen A (2011) Role of efflux pump inhibitors on the antibiofilm efficacy of calcium hydroxide, chitosan nanoparticles, and light-activated disinfection. J Endod 37(10):1422–1426
Distel JW, Hatton JF, Gillespie MJ (2002) Biofilm formation in medicated root canals. J Endod 28(10):689–693
Gomes B et al (2003) Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J 36(4):267–275
Hancock REW (1997) The bacterial outer membrane as a drug barrier. Trends Microbiol 5(1):37–42
Rose RK, Matthews SP, Hall RC (1997) Investigation of calcium-binding sites on the surfaces of selected gram-positive oral organisms. Arch Oral Biol 42(9):595–599
Maye MM et al (2005) Mediator-template assembly of nanoparticles. J Am Chem Soc 127(5):1519–1529
Yoshihisa Y et al (2010) Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp Dermatol 19(11):1000–1006
Onizawa S et al (2009) Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm Pharmacol Ther 22(4):340–349
Rezaei-Zarchi S et al (2012) Study of bactericidal properties of carbohydrate-stabilized platinum oxide nanoparticles. Int Nano Lett 2(1):21
Gopal J et al (2013) Bacterial toxicity/compatibility of platinum nanospheres, nanocuboids and nanoflowers. Sci Rep 3:1260
Rosi NL et al (2006) Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312(5776):1027–1030
Connor EE et al (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1(3):325–327
Esther RJ et al (2005) Gold nanoparticles do not affect the global transcriptional program of human umbilical vein endothelial cells: a DNA-microarray analysis. J Biomed Nanotechnol 1(3):328–335
James FH, Daniel NS, Henry MS (2004) The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 49(18):N309
Taton TA, Mirkin CA, Letsinger RL (2000) Scanometric DNA array detection with nanoparticle probes. Science 289(5485):1757–1760
Gu H et al (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 3(9):1261–1263
Hyland RM et al (2006) N-acetyllactosamine conjugated to gold nanoparticles inhibits enteropathogenic Escherichia coli colonization of the epithelium in human intestinal biopsy specimens. Infect Immun 74(9):5419–5421
Mohamed MM et al (2017) Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int J Vet Sci Med 5(1):23–29
Zhao Y et al (2010) Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J Am Chem Soc 132(35):12349–12356
Brown AN et al (2012) Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol 78(8):2768–2774
Khameneh B et al (2016) Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog 95:32–42
Nazari ZE et al (2012) The combination effects of trivalent gold ions and gold nanoparticles with different antibiotics against resistant Pseudomonas aeruginosa. Gold Bulletin 45(2):53–59
Raafat D et al (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74(12):3764–3773
Helander I et al (2001) Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol 71(2–3):235–244
Goy RC, Britto DD, Assis OB (2009) A review of the antimicrobial activity of chitosan. Polímeros 19(3):241–247
Hayden SC et al (2012) Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J Am Chem Soc 134(16):6920–6923
Zhang Y et al (2008) Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J Colloid Interface Sci 325(2):371–376
Bresee J et al (2011) Growth inhibition of Staphylococcus aureus by mixed monolayer gold nanoparticles. Small 7(14):2027–2031
Bresee J et al (2010) Identification of antibiotics using small molecule variable ligand display on gold nanoparticles. Chem Commun 46(40):7516–7518
Acknowledgements
This study was supported for practical aims by Immunology Research Center (Grant No. 95.5/7.2), Faculty of Medicine, Tabriz University of Medical Sciences, and Tabriz, Iran. This study is a part of Ph.D. thesis of the second author (Thesis No. 5-35638).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasani, A., Madhi, M., Gholizadeh, P. et al. Metal nanoparticles and consequences on multi-drug resistant bacteria: reviving their role. SN Appl. Sci. 1, 360 (2019). https://doi.org/10.1007/s42452-019-0344-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0344-4