Abstract
Graphene, an ideal two dimensional material, has attracted much attention due to its unique structural and physicochemical properties. Herein, we report synthesis of CuO/SiO2 modified amine functionalized reduced graphene oxide (rGO) and its excellent potentialities in environmental remediation and energy storage application. The structure, purity, functional groups and morphology of as-prepared CuO/SiO2:rGO nanocomposites were characterized by XRD, FTIR, FESEM and TEM. The catalytic activity of the CuO/SiO2:rGO in different ratios was evaluated by degradation of methylene blue (MB) under visible light condition. The CuO/SiO2:rGO (mass ratio 1:3) exhibits high catalytic activity with 99% degradation of 20 ppm MB in 7 min. CuO/SiO2:rGO nanocomposites (1:3) also demonstrate good electrochemical performance with specific capacitance of 235 F g−1, which is about fivefold higher than CuO nanoparticles. The nanocomposite (1:3) also reveals excellent cycling stability at 4 A g−1 for 1000 cycles and the capacitance retention was found to be 95% after 1000 cycles. These results validate that the development of a new class of composite necessitates the proper loading of metal oxide and GO to be used for energy and environmental applications.
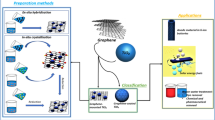
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the trending two dimensional sp2 hybridized carbon materials is graphene. It is inspired in the research community due to its exceptional characteristics such as high conductivity, high theoretical surface area (2600 m2 g−1), high thermal conductivity [1, 2]. The physical properties of graphene which comprises a single atomic layer of carbon atoms arranged in honeycomb network structure. Graphene, being a zero overlap semi-metal with both electrons and holes as charge carriers thereby having high charge carrier mobility 250,000 cm2 V−1 s−1 at room temperature [3]. The other inherent properties of graphene is its mechanical strength with Young’s modulus of 1 TPa and high thermal conductivity (~ 5000 W m−1 k−1). However, the strong cohesive van der Waals force adhering graphitic sheets to one another necessities strong exfoliation of π-stacked graphene sheets. Hence, it requires suitable solution-chemistry based approaches to produce chemically modified graphene. Synthesis of graphene by chemical methods facilitates functionalization with polymeric material, semiconductor metal oxides (SMO’s), biomaterials etc. enabling the modification of surface properties. Chemical based approaches relies on initial oxidation of graphite to graphite oxide, and subsequent exfoliation to graphene oxide. The oxidation and exfoliation causes physical changes in the graphite layers by intercalation, chemical functionalization and thermal expansion, thereby making graphene oxide hydrophilic [4]. However, the physicochemical properties of the nanocomposites depend on the distribution of SMO’s over graphene layers. The functional groups of GO render them to be hydrophilic and enable the decoration of SMO via Van der Waals interaction [4, 5]. Among the various SMO’s, CuO has been investigated for supercapacitor [6, 7], photocatalyst [8] and sensor [9, 10] applications owing to its non-toxicity, abundance and low cost.
CuO is a p-type semiconductor material with a narrow band gap of ~ 1.2–1.5 eV [11, 12]. Beside the development of CuO photocatalyst, the study of supporting material, SiO2 combined to them is also carried out [13]. The chemically inert mesoporous siliceous (SiO2) materials are often employed as a support because they possess high specific surface area, large pore volume, excellent thermal stability and are transparent to UV radiation. To diminish the recombination rate, CuO/SiO2 has to be combined with electron accepting materials such as graphene. Therefore, loading of CuO/SiO2 over graphene is advantageous and could be a good candidate for photocatalyst with improved degradation efficiency [14, 15]. Combination of SiO2 with graphene and other inorganic materials exhibits superior property when compared to bare GO and silica nanoparticles. Combining silica and CuO with graphene tunes the band gap energy levels thereby providing hybrid nanocomposites with highly efficient photocatalytic behaviour.
On the other hand, when CuO is used as an electrode material for supercapacitor, it suffers from poor specific capacitance. To further improve the performance of the supercapacitor, CuO/SiO2 is made as composite with grapheme and offers better performance benefitting from both electric double layer capacitance (EDLC) and pseudocapacitance mechanisms. In addition, incorporation of CuO/SiO2 into graphene layers improves the electrode–electrolyte accessibility and also prevents agglomeration/restacking of graphene layers [6, 16, 17].
In the current study, graphene encapsulated CuO/SiO2 nanocomposites were prepared using electrostatic self-assembly method. A facile synthesis route was instigated in which SiO2 spheres were first synthesized. Then copper chloride was reduced using sodium hydroxide and dextrose over SiO2, followed by amine functionalization and electrostatically self-assembled over reduced graphene oxide sheets. Though there are lots of reports on CuO and graphene based nanocomposites for photocatalytic and supercapacitor applications, the report on combining siliceous material with CuO/graphene by electrostatic self-assembly method is limited [14]. The resulting hybrid nanostructure is electrochemically studied and is found to exhibit good electrochemical performance with excellent cycling stability.
2 Materials and methods
Graphite powder, sodium nitrate (NaNO3), sulphuric acid (H2SO4, 98%), potassium permanganate (KMnO4), hydrogen peroxide (H2O2, 50 wt%), thiourea (SC(NH2)2), ferrous sulphate heptahydrate (FeSO4∙7H2O), 3-aminopropyldimethoxysilane ((CH3O)3 Si(CH2)3NH2, APS), copper chloride dihydrate (CuCl2.2H2O) ammonia (NH3), hydrochloric acid (HCl) deionised water (DI water) was used in the whole experiment.
2.1 Preparation of SiO2 nanospheres
To prepare SiO2 nanospheres, 14.5 mL of aqueous ammonia and 76 mL of absolute ethanol were added to 50 mL of distilled water containing 2.5 g of n-hexadecyltrimethylammonium bromide under magnetic stirring for 15 min. It was followed by the addition of 5 mL of triethylorthosilicate (TEOS) and continuously stirred for 2 h. The resulting white precipitate was filtered and washed with distilled water and ethanol (2 × 10 mL). After drying under vacuum overnight, the samples were calcined at 500 °C for 5 h.
2.2 Preparation of CuO/SiO2 composite
To prepare CuO/SiO2 nanospheres, 0.34 g of calcined SiO2 was dispersed in 100 mL of distilled water at 60 °C for 30 min. To the above solution, 0.34 g of CuCl2.2H2O was added and stirred for 15 min. It was followed by the dropwise addition of 0.2 M of NaOH until pH of 11. Simultaneously, 0.108 g of dextrose in 30 mL distilled water was added and continued to be stirred for 30 min at 60 °C. The resulting precipitate was centrifuged and washed with water several times (3 × 10 mL). After drying under vacuum overnight, the samples were calcined at 500 °C for 3 h. The similar procedure was followed to prepare CuO, but without the addition of SiO2.
2.3 Preparation of amine modified CuO/SiO2 composites
Amine modification was carried out by measuring 0.1 g of CuO/SiO2 in 50 mL isopropanol followed by the addition of 3-aminopropyl tri-methoxysilane (2 mL) and heated at 80 °C for 24 h. Finally the samples were centrifuged, dried in vacuum and dried at 60 °C for 1 h.
2.4 Synthesis of CuO/SiO2:rGO nanocomposites
GO was prepared by modified Hummers method [18,19,20,21]. In the preparation process, 50 mg of amine modified CuO/SiO2 was sonicated for few minutes in 50 mL of distilled water. To the above solution, 50 mg mL−1 of GO was added and heated at 80 °C. Then 1.5 g of urea was added and continued to be heated at 80–90 °C for 4 h. The resulting solution was washed with water several times (3 × 10 mL) and allowed to dry at 60 °C overnight, the product being labelled 1:1 (CuO/SiO2:rGO). Similar experimental procedure was adopted to prepare other variable ratios of CuO/SiO2 and GO (CuO/SiO2:rGO in 1:2 (50 mg (CuO/SiO2): 100 mg GO), 1:3 (50 mg (CuO/SiO2): 150 mg GO), 2:1 (100 mg (CuO/SiO2): 50 mg GO), 3:1 (150 mg (CuO/SiO2): 50 mg GO) respectively).
2.5 Materials characterization
Crystalline nature was analyzed using Xpert-Pro diffractometer with Cu-Kα radiation (λ = 1.54060 Å) in the 2θ range between 5° and 90°. UV–visible diffuse reflectance spectra (DRS) analysis was conducted using UV–Vis spectrophotometer and reflectance was converted to absorbance by Kubelka–Munk function. Raman studies were carried at room temperature using Jobin–Yvon spectrometer using 514 nm argon lasers. FT-IR spectra of the samples were collected using ATR-FTIR (Bruker, Germany). The morphology of the samples were analyzed using field emission scanning electron microscopy (FE-SEM) with a FEI Quanta 3D.
2.6 Photocatalytic activity
The typical photocatalytic activity test was carried out under visible light condition on sunny days of October 2017 in the city of Tiruchirappalli (geographical location 10°39′26″N 78°44′46″E) between 10 am to 4 pm. All the photocatalytic degradation experiments were carried out with 20 mg of catalyst and the concentration of methylene blue was fixed as 20 ppm. Prior to irradiation, above mentioned solution mixture was stirred in dark for 2 min to obtain adsorption/desorption equilibrium after which the reaction solution was kept under visible light. Sample solution of 2 mL was syringed out at regular time intervals and supernatant was filtered. The concentration of the dye in the solution was measured as the function of time. UV–visible spectrophotometer was used to analyse the degradation of dye solution as the function of concentration.
2.7 Electrochemical measurement
The electrochemical performance of the prepared electrode material was investigated by cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) techniques on an electrochemical workstation (Metrohm, Netherlands) using a three compartment cell at ambient temperature. The synthesized electrode materials, platinum wire and Ag/AgCl were used as working, counter and reference electrodes, respectively. The working electrodes were prepared by mixing active material (70%), acetylene black (20%) and PVDF (polyvinylidene fluoride) (10%) as a binder in agate mortar. N-methyl-2-pyrrolidene (NMP) was used as a solvent. The homogenous slurry was coated on carbon felt (1 cm × 1 cm). The resulting samples were dried at 80 °C for 12 h. All electrochemical measurements were done in 1 M H2SO4 electrolyte.
3 Results and discussion
3.1 X-ray diffraction analysis
The structure, phase and purity of CuO and CuO/SiO2:rGO nanocomposites were investigated by X-ray diffraction analysis (Fig. 1). The diffraction peaks match well with monoclinic crystal system with C2/C space group (JCPDS no: 00-002-1040). No other peaks corresponding to impurities and SiO2 were observed in CuO/SiO2 nanocomposite preparation. The diffraction intensities of CuO/SiO2 are found to be sharper and intense than CuO, which implies the formation of larger CuO/SiO2 nanostructures than CuO.
The influence of calcination over the formation of CuO and CuO/SiO2 nanostructures was also examined by X-ray diffraction (Fig. S1). When CuO/SiO2 nanocomposites were surface modified with APS, the diffraction peak intensities were decreased, in which positively charged NH3+ groups anchored in the surface of CuO/SiO2, which consequently resulted in the reduction in the size of nanostructures. There are no additional peaks in the diffraction pattern obtained; rather there is change in the diffraction intensities when CuO/SiO2 nanostructure is made composite with variable ratios of GO. In CuO/SiO2:rGO (mass ratio of 1:3), the major diffraction intensities of CuO is reduced completely, which ensures that CuO/SiO2 nanostructures are anchored on rGO sheets. In other CuO/SiO2:rGO mass ratio composites, there is not much decrease in the diffraction intensities.
3.2 Raman analysis
Raman spectroscopy is a sensitive probe to analyze the vibrations, local atomic arrangements of the material and also used to characterize the structural information. CuO, which is found to be in monoclinic structure from the XRD data has two molecules per primitive cell with C 62h space group comprises 12 vibrational modes such as acoustic modes (one Au and two Bu), six infrared active modes (three Au and three Bu) and three Raman active modes (one Ag and two Bg) [8, 22,23,24]. These lattice vibrations at the Г point of the Brillouin zone has been given based on the following group theory (Eq. 1).
The presence of Raman active modes in the composites was confirmed by the Raman spectroscopy technique (Fig. 2). Raman spectra of CuO exhibits a peak at 282 cm−1 which is characteristic of Ag mode, and the peaks at 340 and 620 cm−1 are assigned to Bg mode [25]. The dominant Ag mode (340 cm−1) of CuO in CuO/SiO2, which makes clear that CuO nanoflakes are distributed over the surface of SiO2 spheres as evident from the microscopic images. The raman spectrum of CuO/SiO2:rGO nanocomposite shows D band at 1350 cm−1 and G band at 1595 cm−1, which represent sp3 and sp2 carbon vibrations in graphitic lattice, respectively [26,27,28]. These bands are the characteristic peaks of graphene and the intensity varies upon various loadings of GO.
3.3 Microscopic studies
The surface morphologies of CuO and CuO/SiO2:rGO nanocomposites were investigated by SEM analysis. Figure 3a shows that CuO was formed as thin flaky structure and it was evident that the flakes were nanosized. When CuO precursor was added to SiO2, initially it resulted in the formation of SiO2 spheres followed by the CuO flaky structure covering the SiO2 spheres (Fig. 3b). Figure 3c–h demonstrates the dispersion of CuO/SiO2 over rGO sheets for the variable ratios of CuO/SiO2 and GO added. Further insights into the morphological characteristics of CuO/SiO2:rGO (1:3) were investigated by TEM (Fig. 4). The TEM images suggest that rGO has a few stacks of layers. It was also found to be transparent and thin with the dispersion of CuO/SiO2, which is in good harmony with FESEM results.
3.4 UV–visible diffuse reflectance spectra analysis
Besides, the structural, functional and morphological studies, the optical properties of the composites are also studied which are highly important for photocatalytic applications. From the UV–visible diffuse reflectance spectra (Fig. 5), the band gap values can be calculated by using Kubelka–Munk function [14, 29].
where R(hν) is the absolute reflectance and F[R(hν)] represents the Kubelka–Munk function. From F[R(hν)], the band gap energy values can be directly calculated by the following equation.
where n = 1/2 for direct and n = 2 for indirect electron transition, A represents the proportionality constant. Finally, the plots were made between [F(R(hν))*hν]1/2 versus (hν) to evaluate the band gap (Fig. 5 inset). The calculated band gap energy value of CuO was ca. 1.29 eV. The band gap energy values decreased linearly with the increase in GO content, which clearly suggests that the prepared nanocomposites are compatible for visible light absorption [30].
3.5 Photocatalytic activity
The photocatalytic activity of CuO, rGO and CuO/SiO2:rGO nanocomposites were examined by degradation of MB. The degradation experiments were carried out using 20 ppm of MB solution at normal room conditions under visible light irradiation. In all the experiments, 20 mg of catalyst was used. The CuO/SiO2:rGO composite (1:3) with higher GO content has shown to exhibit immediate degradation as shown in Fig. 6. It can be seen that the intensity of MB at 664 nm gradually decreases with respect to reaction time and CuO/SiO2:rGO composite (1:3) took only 7 min to completely degrade the dye (~ 100%) (Fig. 6c, f). The catalytic activities of the nanocomposite were in the following order: CuO/SiO2:rGO (1:3) [7 min] {99%} > (1:2) [283 min] {88.6%} > (1:1) [260 min] {61.45%} > (2:1) [150 min] {46.22%} > (3:1) [90 min] {23.53%} beyond which there was a negligible degradation of MB. The superior photocatalytic activity of CuO/SiO2:rGO (1:3) nanocomposite is due to the interaction between graphene and CuO, where the GO content is higher. Graphene is well known to be an electron acceptor with a work function of about 4.2 eV. The conduction and valence band edges of CuO lies in 0.39 eV and 2.22 eV. When photon with higher energy than the band gap of CuO is illuminated, the electrons from the valence band of CuO move to the conduction band, leaving a hole behind in the valence band. The conduction band electrons of CuO are transferred to graphene and the retardation of photogenerated electron–hole recombination takes place [15, 31].
To examine the role of CuO/SiO2 in dye degradation, we increased the amount of CuO/SiO2 to GO (2:1 and 3:1). Under similar experimental conditions, the 2:1 ratio took 86 min and 3:1 ratio took 102 min to exhibit 94% and 92% degradation of MB, respectively. This time and percentage of degradation difference shows that composite with sufficient amount of GO exhibits an enhanced photocatalytic activity. It is noteworthy to mention that insufficient amount of GO leads to recombination of photogenerated electrons and holes leading to reduced photocatalytic activity [31, 32]. The degradation of MB was also examined by using only rGO and found to exhibit lower degradation efficiency (Fig. 7b). The excessive amount of dye molecules attached to the rGO surface may hinder the photon from reaching the surface of rGO resulting in the lower degradation efficiency (Fig. 8).
3.6 Electrochemical studies
To evaluate the electrochemical performance of nanocomposites, cyclic voltammetry and galvanostatic charge–discharge measurements were carried out in 1 M H2SO4. The CV curves of CuO/SiO2:rGO (1:3) nanocomposite are nearly rectangular which clearly indicates that the specific capacitance is dominated by EDLC and less of that of pseudocapacitance (Fig. 9); whereas for CuO, the specific capacitance is attributed to the pseudocapacitive behaviour [16, 33]. CV curves of CuO nanostructures show two cathodic peaks (positive current) around + 0.34 V at a scan rate of 100 mV, where the maximum flow of electrons is observed and reduction of Cu+/Cu takes place at + 0.52 V. Beyond this point, the current associated with the reduction decreases due to the slower diffusion of CuO than reduction. Additionally, the peak potentials were shifted with the scan rate < 100 mV s−1, which is mainly associated to the internal resistance of the electrode. It could also be seen that the cathodic peak current linearly increases with the square root of scan rate (scan rate < 50 mV s−1) which indicates that at lower scan rates, diffusion controlled reaction takes place. There was a little increase in the peak current obtained beyond the scan rate of 50 mV s−1.
The larger area of CV curves of CuO/SiO2:rGO (1:3) nanocomposite indicates that its specific capacitance is higher than that of other ratios of nanocomposite. All the nanocomposites show nearly symmetrical curves even at higher scan rates, which clearly indicate good propagation within the electrode. The specific capacitance values were calculated from the obtained CV curves using the following equation:
Where ∫ Idv is the integral area of CV curve, v is the scan rate, m is the mass of the active material, ∆V is the potential window. The variation of specific capacitance with respect to scan rate is shown in Fig. 10. CuO/SiO2:rGO (1:3) nanocomposite exhibits specific capacitance of 237.028 F g−1 at 10 mV s−1. It could be seen that at higher scan rate, CuO/SiO2:rGO (1:3) nanocomposite shows lower capacitance mainly due to electrolyte ions not being provided enough time to access the electrode surface, which is in contrast to lower scan rate results [34, 35]. Furthermore, there is obvious increase in the current with increasing scan rate which describes the rate capability of the electrode material.
The galvanostatic charge/discharge method is another complementary method for evaluating the electrochemical performance of the electrode material at controlled current conditions. CuO shows nearly a triangle shape with symmetry indicating its good electrochemical behaviour (Fig. 11). The specific capacitance of the electrode was calculated from the obtained charge/discharge data by using the following equation:
where I is the constant discharging current, ∆t is the discharging time, ∆V is the potential window and m is the mass of the active material in the electrode. The specific capacitance of CuO/SiO2:rGO (1:3) nanocomposite was found to be 234.08 F g−1, which is ~ 5.85 fold higher than CuO. This higher capacitance value indicates that the higher ratio of GO added to CuO/SiO2 provided a conductive support and also rendered homogeneous distribution of CuO over rGO sheets [36]. Notably, there is a strong bonding between CuO and rGO enabling a short electron transportation and ion diffusion pathway leading to good rate capability [37, 38].
To investigate the electrochemical stability of CuO/SiO2:rGO (1:3) nanocomposite, which showed higher specific capacitance than other ratios of composite, we preformed charge/discharge experiment at 4 A g−1 for 1000 cycles. As shown in Fig. 12, the capacitance was retained at 114 F g−1 (95% of initial capacitance) even after 1000 cycles which is much higher than initial specific capacitance of CuO (63.69 F g−1) at 4 A g−1. This shows that CuO/SiO2:rGO (1:3) nanocomposite exhibits higher specific capacitance and remarkable electrochemical stability as an electrode for high performance supercapacitor. The good electrochemical performance of the CuO/SiO2:rGO (1:3) nanocomposite may be ascribed to the presence of CuO nanostructures, which prevented the aggregation/stacking of graphene sheets.
4 Conclusion
Nanostructured CuO/SiO2:rGO hybrid composites were synthesized by a simple low temperature wet-chemical method. The X-ray diffraction pattern confirms that CuO/SiO2:rGO nanocomposites are in monoclinic crystal system with C2/C space group. The Raman spectrum of CuO/SiO2:rGO nanocomposite corroborates the sp3 and sp2 carbon vibrations in graphitic lattice. Microscopic analyses results demonstrate the dispersion of CuO/SiO2 over rGO sheets. Investigations on photocatalytic degradation of MB reveal that CuO/SiO2:rGO (1:3) nanocomposites afford complete degradation of MB in 7 min. The electrochemical studies suggest that the synergistic effect enables the CuO/SiO2:rGO (1:3) nanocomposite to exhibit excellent electrochemical performance with high specific capacitance and good cycling stability even after 1000 cycles. In principle, these findings suggests that CuO/SiO2:rGO nanocomposites were found to be an excellent catalyst for the degradation of organic dye pollutant under visible light and a promising candidate for electrochemical capacitor electrode material.
References
Deng S, Berry V (2016) Wrinkled, rippled and crumpled graphene: an overview of formation mechanism, electronic properties, and applications. Mater Today 19:197–212
Zhang H, Lv X, Li Y, Wang Y, Li J (2010) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small 6:711–723
Chen D, Li J, Chen D (2010) Graphene-based materials in electrochemistry. Chem Soc Rev 39:3157–3180
Chen D, Zhang H, Liu Y, Lia J (2013) Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications Energ. Environ Sci 6:1362–1387
Wang K, Dong X, Zhao C, Qian X, Xu Y (2015) Facile synthesis of Cu2O/CuO/RGO nanocomposite and its superior cyclability in supercapacitor. Electrochim Acta 152:433–442
Li Y, Ye K, Cheng K, Cao D, Pan Y, Kong S, Zhang X, Wang G (2014) Anchoring CuO nanoparticles on nitrogen-doped reduced graphene oxide nanosheets as electrode material for supercapacitors. J Electroanal Chem 727:154–162
Mukherjee N, Show B, Maji SK, Madhu U, Bhar SK, Mitra BC, Khan GG, Mondal A (2011) CuO nano-whiskers: electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity Nillohit. Mater Lett 65:3248–3250
Yang J, Tan W, Chen C, Tao Y, Qin Y, Kong Y (2017) Nonenzymatic glucose sensing by CuO nanoparticles decorated nitrogen-doped graphene aerogel. Mater Sci Eng C 78:210–217
Lee C, Lee SH, Cho M, Lee Y (2016) Nonenzymatic amperometric glucose sensor based on a composite prepared from CuO, reduced graphene oxide, and carbon nanotube. Microchim Acta 183:3285–3292
Dar MA, Kim YS, Kim WB, Sohn JM, Shin HS (2008) Structural and magnetic properties of CuO nanoneedles synthesized by hydrothermal method. Appl Surf Sci 254:7477–7481
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7:70
Mohammadzadeh R, Kakhki F, Ahsani N Mir (2016) Enhanced photocatalytic activity of CuO–SiO2 nanocomposite based on a new Cu nanocomplex. J Mater Sci Mater Electron 27:11509–11517
Nguyen DCT, Oh WC (2018) Ternary self-assembly method of mesoporous silica and Cu2O combined graphene composite by nonionic surfactant and photocatalytic degradation of cationic–anionic dye pollutants. Sep Purif Technol 190:77–89
Choi J, Oh H, Han S-W, Ahn S, Noh J, Park JB (2016) Preparation and characterization of graphene oxide supported Cu, Cu2O, and CuO nanocomposites and their high photocatalytic activity for organic dye molecule. Curr Appl Phys 17:137–145
Bu IYY, Huang R (2017) Fabrication of CuO-decorated reduced graphene oxide nanosheets for supercapacitor applications. Ceram Int 43:45–50
He Z, Zhu Y (2014) Reduced graphene oxide–cuprous oxide hybrid nanopowders: solvothermal synthesis and electrochemical performance. Mater Sci Semicond Proc 27:1013–1019
Singh RK, Kumar R, Singh DP (2016) Graphene oxide: strategies for synthesis, reduction and frontier applications. RSC Adv 6:64993–65011
Bhoyate S, Mensah-Darkwa K, Kahol PK, Gupta RK (2017) Recent development on nanocomposites of graphene for supercapacitor applications. Curr Graphene Sci 1:1
Wang G, Shen X, Yao J, Park J (2009) Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 47:2049–2053
Luo G, Huang H, Lei C, Cheng Z, Wu X, Tang S, Du Y (2016) Facile synthesis of porous graphene as binder-free electrode for supercapacitor application. Appl Surf Sci 366:46–52
Levitskii VS, Shapovalov VI, Komlev AE, Zav’yalov AV, Vit’ko VV, Komlev AA, Shutova ES (2015) Raman spectroscopy of copper oxide films deposited by reactive magnetron sputtering. Tech Phys Lett 41:1094–1096
Hagemam H, Bill H, Sadowski W, Walker E, Francois M (1990) Raman spectra of single crystal CuO. Solid State Commun 73:447–451
Xu JF, Ji W, Shen ZX, Tang SH (1999) Preparation and characterization of CuO nanocrystals. J Solid State Chem 147:516–519
Rashad M, Rüsing M, Berth G, Lischka K, Pawlis A (2013) CuO and Co3O4 nanoparticles: synthesis, characterizations, and raman spectroscopy. J Nanomater 2013:6
Wang L, Zhao J, Sun YY, Zhang SB (2011) Characteristics of Raman spectra for graphene oxide from ab initio simulations. J Chem Phys 135:184503
Pendashteh A, Fazlollah M, Safi M (2013) Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor. Electrochim Acta 88:347–357
Liu S, Tian J, Wang L, Luo Y, Sun X (2012) One-pot synthesis of CuO nanoflower-decorated reduced graphene oxide and its application to photocatalytic degradation of dyes Catal. Sci Technol 2:339–344
Rehman S, Mumtaz A, Hasanain SK (2011) Size effects on the magnetic and optical properties of CuO nanoparticles. J Nanopart Res 13:2497–2507
Veldurthi NK, Palla S, Velchuri R, Guduru P, Muga V (2015) Degradation of mixed dyes in aqueous wastewater using a novel visible light driven LiMg0.5Mn0.5O2 photocatalyst. Mater Express 5:445–450
Zeng B, Long H (2016) Graphene spheres-CuO nanoflowers composites for use as a high performance photocatalyst. Nanomater Nanotechnol 6:21
Low J, Cao S, Yu J, Wageh S (2014) Two-dimensional layered composite photocatalysts. Chem Commun 50:10768
Wang S, ** S, Wang X (2011) Microwave-assisted one-pot synthesis of metal/metal oxide nanoparticles on graphene and their electrochemical applications. Electrochim Acta 56:3338–3344
Lee JSM, Briggs ME, Hu CC, Cooper AI (2018) Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers. Nano Energy 46:277–289
Sharma V, Singh I, Chandra A (2018) Hollow nanostructures of metal oxides as next generation electrode materials for supercapacitors. Sci Rep 8:1–12
Ke Q, Wang J (2016) Graphene-based materials for supercapacitor electrodes—a review. J Materiomics 2:37–54
Parwaiz S, Bhunia K, Das AK, Khan MM, Pradhan D (2017) Cobalt-doped ceria/reduced graphene oxide nanocomposite as an efficient oxygen reduction reaction catalyst and supercapacitor material. J Phys Chem C 121:20165–20176
Chen YL, Hu ZA, Chang YQ, Wang HW, Zhang ZY, Yang YY, Wu HY (2011) Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J Phys Chem C 115:2563–2571
Acknowledgements
The author PJS acknowledges the receipt of fellowship from TEQIP-II, BIT campus. KN and MS acknowledge the CSIR for partial funding. PB acknowledges CSIR-HRDG for providing support under Scientist Pool Scheme (No: 13(8778-A)/2015-Pool). TDT acknowledges CSIR for financial support (No. 01(2818)/14/EMR-II). The authors acknowledge SNR Sons and Charitable Trust, Coimbatore, for electrochemical work station and XRD characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sephra, P.J., Baraneedharan, P., Sivakumar, M. et al. CuO/SiO2 modified amine functionalized reduced graphene oxide with enhanced photocatalytic and electrochemical properties. SN Appl. Sci. 1, 73 (2019). https://doi.org/10.1007/s42452-018-0074-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-018-0074-z