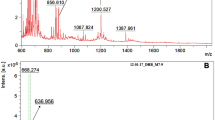
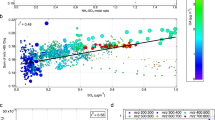
Abstract
The ozonolysis of cyclohexene is an important model system for understanding the more complex reaction of O3 with monoterpenes; however, many previous studies have come to qualitatively different conclusions about the composition of the secondary organic aerosol (SOA) formed in this system. In the present study, the composition of the SOA produced by cyclohexene ozonolysis in the absence of seed aerosols has been investigated online and off-line using synchrotron-based thermal desorption/tunable vacuum ultraviolet photoionization time-of-flight aerosol mass spectrometry (TD-VUV-TOF-PIAMS) in conjunction with a custom-built smog chamber. On the basis of the molecular ions observed by mass spectrometry at 11.5 eV, it was found that dicarboxylic acids, dialdehydes, and cyclic anhydrides are the predominant low molecular weight components in the particle phase. The results also indicated that TD-VUV-TOF-PIAMS coupled with filter sampling is a potentially useful tool for the investigation of SOA composition both in the field and in the laboratory.




Similar content being viewed by others
References
M.P. Fraser, G.R. Cass, B.R.T. Simoneit, Gas-phase and particle-phase organic compounds emitted from motor vehicle traffic in a Los Angeles roadway tunnel. Environ. Sci. Technol. 32, 2051–2060 (1998). https://doi.org/10.1021/es970916e
R.J. Griffin, D.R. Cocker, R.C. Flagan et al., Organic aerosol formation from the oxidation of biogenic hydrocarbons. J. Geophys. Res. 104, 3555–3567 (1999). https://doi.org/10.1029/1998JD100049
B.R. Larsen, D.D. Bella, M. Glasius, R. Winterhalter et al., Gas-phase OH oxidation of monoterpenes: gaseous and particulate products. J. Atmos. Chem. 38, 231–276 (2001). https://doi.org/10.1023/A:1006487530903
S. Hatakeyama, T. Tanonaka, J.H. Weng et al., Ozone-cyclohexene reaction in air: quantitative analysis of particulate products and the reaction mechanism. Environ. Sci. Technol. 19, 935–942 (1985). https://doi.org/10.1021/es00140a008
M. Kalberer, J. Yu, D.R. Cocker et al., Aerosol formation in the cyclohexene-ozone system. Environ. Sci. Technol. 34, 4894–4901 (2000). https://doi.org/10.1021/es001180f
P.J. Ziemann, Evidence for low-volatility diacyl peroxides as a nucleating agent and major component of aerosol formed from reactions of O3 with cyclohexene and homologous compounds. J. Phys. Chem. A 106, 4390–4402 (2002). https://doi.org/10.1021/jp012925m
S.M. Aschmann, E.C. Tuazon, J. Arey et al., Products of the gas-phase reaction of O3 with cyclohexene. J. Phys. Chem. A 107, 2247–2255 (2003). https://doi.org/10.1021/jp022122e
J.F. Hamilton, A.C. Lewis, J.C. Reynolds et al., Investigating the composition of organic aerosol resulting from cyclohexene ozonolysis: low molecular weight and heterogeneous reaction products. Atmos. Chem. Phys. 6, 4973–4984 (2006). https://doi.org/10.5194/acp-6-4973-2006
M. Narukawa, Y. Matsumi, J. Matsumoto et al., Real-time analysis of secondary organic aerosol particles formed from cyclohexene ozonolysis using a laser-ionization single-particle aerosol mass spectrometer. Anal. Sci. 23, 507–512 (2007). https://doi.org/10.2116/analsci.23.507
M.P. Rissanen, T. Kurtén, M. Sipilä et al., The formation of highly oxidized multifunctional products in the ozonolysis of cyclohexene. J. Am. Chem. Soc. 136, 15596–15606 (2014). https://doi.org/10.1021/ja507146s
W.F. Rogge, M.A. Mazurek, L.M. Hildemann et al., Quantification of urban organic aerosols at a molecular level: identification, abundance and seasonal variation. Atmos. Environ. Part A 27, 1309–1330 (1993). https://doi.org/10.1016/0960-1686(93)90257-y
B.J. Williams, A.H. Goldstein, D.B. Millet et al., Chemical speciation of organic aerosol during the international consortium for atmospheric research on transport and transformation 2004: results from in situ measurements. J. Geophys. Res. (Atmos.) 112, D10S26 (2007). https://doi.org/10.1029/2006jd007601
E.R. Mysak, K.R. Wilson, M. Jimenez-Cruz et al., Synchrotron radiation based aerosol time-of-flight mass spectrometry for organic constituents. Anal. Chem. 77, 5953–5960 (2005). https://doi.org/10.1021/ac050440e
M.T. Baeza-Romero, F. Gaie-Levrel, A. Mahjoub et al., A smog chamber study coupling a photoionization aerosol electron/ion spectrometer to VUV synchrotron radiation: organic and inorganic-organic mixed aerosol analysis. Eur. Phys. J. D 70, 154 (2016). https://doi.org/10.1140/epjd/e2016-70264-8
W.Z. Fang, L. Gong, X.B. Shang et al., Thermal desorption/tunable vacuum-ultraviolet time-of-flight photoionization aerosol mass spectrometry for investigating secondary organic aerosols in chamber experiments. Anal. Chem. 83, 9024–9032 (2011). https://doi.org/10.1021/ac201838e
E. Gloaguen, E.R. Mysak, S.R. Leone et al., Investigating the chemical composition of mixed organic–inorganic particles by “soft” vacuum ultraviolet photoionization: the reaction of ozone with anthracene on sodium chloride particles. Int. J. Mass Spectrom. 258, 74–85 (2006). https://doi.org/10.1016/j.ijms.2006.07.019
N.N. Wei, C.J. Hu, S.S. Zhou et al., VUV photoionization aerosol mass spectrometric study on the iodine oxide particles formed from O3-initiated photooxidation of diiodomethane (CH2I2). RSC Adv 7, 56779 (2017). https://doi.org/10.1039/c7ra11413c
G. Pan, C.J. Hu, Z.Y. Wang et al., Direct detection of isoprene photooxidation products by using synchrotron radiation photoionization mass spectrometry. Rapid Commun. Mass Spectrom. 26, 189–194 (2011). https://doi.org/10.1002/rcm.5295
W.Z. Fang, L. Gong, Q. Zhang et al., Measurements of secondary organic aerosol formed from OH-initiated photo-oxidation of isoprene using online photoionization aerosol mass spectrometry. Environ. Sci. Technol. 46, 3898–3904 (2012). https://doi.org/10.1021/es204669d
W.Z. Fang, L. Gong, X.B. Shan et al., A VUV photoionization organic aerosol mass spectrometric study with synchrotron radiation. J. Electron Spectrosc. Relat. Phenom. 184, 129–133 (2011). https://doi.org/10.1016/j.elspec.2010.12.004
S.S. Wang, R.H. Kong, X.B. Shan et al., Performance of the atomic and molecular physics beamline at the National Synchrotron Radiation Laboratory. J. Synchrotron Rad. 13, 415–420 (2006). https://doi.org/10.1107/S0909049506030536
A.W. Ray, C.A. Taatjes, O. Welz et al., Synchrotron photoionization measurements of OH-initiated cyclohexene oxidation: ring-preserving products in OH + cyclohexene and hydroxycyclohexyl + O2 reactions. J. Phys. Chem. A 116, 6720–6730 (2012). https://doi.org/10.1021/jp3022437
K. Sato, Chemical compositions of secondary organic aerosol from the ozonolysis of cyclohexene in the absence of seed particles. Chem. Lett. 34, 1584–1585 (2005). https://doi.org/10.1246/cl.2005.1584
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the National Natural Science Foundation of China (Nos. 11575178, 91544105, U1532137, 91544228, and U1232130).
Rights and permissions
About this article
Cite this article
Chen, J., Li, ZH., Yu, YP. et al. A reinvestigation of low molecular weight components in SOA produced by cyclohexene ozonolysis. NUCL SCI TECH 29, 162 (2018). https://doi.org/10.1007/s41365-018-0491-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-018-0491-0