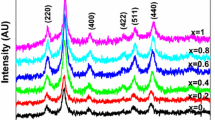
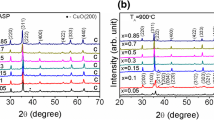
Abstract
Monodisperse nonstoichiometric zinc ferrite nanoparticles with a tunable size of 4.1–32.2 nm are fabricated via thermal decomposition. An extrinsic impurity phase of the ZnO component is present in the zinc ferrite nanoparticles with a size of <10 nm, but this phase can be eliminated after the air annealing treatment. The atom ratio of Zn/Fe and concentration of oxygen vacancies decrease as the particle size of zinc ferrite increases, causing magnetic transition from superparamagnetism to ferromagnetism. The X-ray magnetic circular dichroism spectra reveal that the spin magnetic moments of Fe3+ are reduced, and the orbital magnetic moments are frozen with the increasing atom ratio of Zn/Fe. Therefore, saturation magnetization decreases. The saturation magnetizations of all the zinc ferrite nanoparticles decrease after the air annealing treatment, suggesting that oxygen vacancies considerably influence the magnetic properties. The air annealing treatment can minimize the number of oxygen defects, which trigger some of the Fe3+-OV-Fe3+ ferrimagnetic couplings to transfer into the Fe3+-O2−-Fe3+ antiferromagnetic couplings. This work provides new insights regarding the magnetic performance of spinel ferrites by tuning the stoichiometric ratio and oxygen defects.
摘要
本文采用热解法制备了粒径在4.1–32.2 nm范围内可调的单分散非化学计量比锌铁氧体纳米颗粒. 当颗粒尺寸小于10 nm时, 样品中含有少量的非本征ZnO杂质相, 且空气退火后该杂质相消失. 锌铁氧体中锌/铁原子比与氧缺陷浓度均随着颗粒尺寸的增大而降低, 导致其从超顺磁性转变为铁磁性. 磁性圆二色谱表明, 随着Zn/Fe比的增加, Fe3+的自旋磁矩减小, 轨道磁矩冻结, 饱和磁化**度降低. 经过空气退火, 所有样品的饱和磁化**度降低, 表明氧缺陷(OV)对其磁性有很大影响. 空气退火会降低氧缺陷含量, 部分Fe3+–OV–Fe3+铁磁耦合转变为Fe3+–O2−–Fe3+反铁磁耦合. 该工作通过调控化学计量比和氧缺陷实现了对锌铁氧体磁性的调节, 为理解和调控铁氧体的磁学性质提供了新的思路.
Similar content being viewed by others
References
**ong QQ, Tu JP, Shi SJ, et al. Ascorbic acid-assisted synthesis of cobalt ferrite (CoFe2O4) hierarchical flower-like microspheres with enhanced lithium storage properties. J Power Sources, 2014, 256: 153–159
Bigham A, Foroughi F, Motamedi M, et al. Multifunctional nanoporous magnetic zinc silicate-ZnFe2O4 core-shell composite for bone tissue engineering applications. Ceramics Int, 2018, 44: 11798–11806
Liao W, Zhou G. Conditions for magnetic and electronic properties of ultrathin Ni-Fe hydroxide nanosheets as catalysts: a DFT+U study. Sci China Mater, 2017, 60: 664–673
Daffé N, Gavrilov V, Neveu S, et al. Small CoFe2O4 magnetic nanoparticles in ferrofluids, influence of the synthesis on the magnetic anisotropies. J Magn Magn Mater, 2019, 477: 226–231
Zheng X, Feng J, Zong Y, et al. Hydrophobic graphene nanosheets decorated by monodispersed superparamagnetic Fe3O4 nanocrystals as synergistic electromagnetic wave absorbers. J Mater Chem C, 2015, 3: 4452–4463
He DX, Qiu Y, Li LL, et al. Large-scale solvent-thermal synthesis of graphene/magnetite/conductive oligomer ternary composites for microwave absorption. Sci China Mater, 2015, 58: 566–573
Maiti D, Saha A, Devi PS. Surface modified multifunctional ZnFe2O4 nanoparticles for hydrophobic and hydrophilic anticancer drug molecule loading. Phys Chem Chem Phys, 2016, 18: 1439–1450
Wang S, Sun Z, Hou Y. Engineering nanoparticles toward the modulation of emerging cancer immunotherapy. Adv Healthcare Mater, 2020, 9: 2000845
Zhu K, Ju Y, Xu J, et al. Magnetic nanomaterials: chemical design, synthesis, and potential applications. Acc Chem Res, 2018, 51: 404–413
Morrish AH. The Physical Principle of Magnetism. New York: IEEE Press, 2001
Dionne GF. Magnetic Oxides. New York: S**er-Verlag, LLC, 2009
Kodama RH, Berkowitz AE, McNiff Jr. EJ, et al. Surface spin disorder in ferrite nanoparticles. Mater Sci Forum, 1996, 235–238: 643–650
Yadav RS, Havlica J, Masilko J, et al. Structural, cation distribution, and magnetic properties of CoFe2O4 spinel ferrite nanoparticles synthesized using a starch-assisted sol-gel auto-combustion method. J Supercond Nov Magn, 2015, 28: 1851–1861
Yang Y, Liu X, Yang Y, et al. Synthesis of nonstoichiometric zinc ferrite nanoparticles with extraordinary room temperature magnetism and their diverse applications. J Mater Chem C, 2013, 1: 2875
Nath BK, Chakrabarti PK, Das S, et al. Mössbauer, X-ray diffraction and AC susceptibility studies on nanoparticles of zinc substituted magnesium ferrite. Eur Phys J B, 2004, 39: 417–425
Smit J, Wijn HPJ. Ferrites. Philips Technical Library, Eindhoven, Netherland, 1959
Schiessl W, Potzel W, Karzel H, et al. Magnetic properties of the ZnFe2O4 spinel. Phys Rev B, 1996, 53: 9143–9152
Yao C, Zeng Q, Goya GF, et al. ZnFe2O4 nanocrystals: Synthesis and magnetic properties. J Phys Chem C, 2007, 111: 12274–12278
Néel L. Superparamagnétisme des grains très fins antiferromagnétiques. Compt Rend, 1961, 252: 4075
Yelenich OV, Solopan SO, Kolodiazhnyi TV, et al. Magnetic properties and high heating efficiency of ZnFe2O4 nanoparticles. Mater Chem Phys, 2014, 146: 129–135
Fang Z, Zhang L, Qi H, et al. Nanosheet assembled hollow ZnFe2O4 microsphere as anode for lithium-ion batteries. J Alloys Compd, 2018, 762: 480–487
Zhang Y, Wu Y, Qin Q, et al. A study of the mechanism of microwave-assisted ball milling preparing ZnFe2O4. J Magn Magn Mater, 2016, 409: 6–9
Ismail M, Hao A, Huang W, et al. Coexistence of unipolar and bipolar switching in nanocrystalline spinel ferrite ZnFe2O4 thin films synthesized by sol-gel method. Appl Phys Lett, 2018, 113: 152103
Park J, Porter MD, Granger MC. Silica encapsulation of ferrimagnetic zinc ferrite nanocubes enabled by layer-by-layer polyelectrolyte deposition. Langmuir, 2015, 31: 3537–3545
Rozman M, Drofenik M. Hydrothermal synthesis of manganese zinc ferrites. J Am Ceramic Soc, 1995, 78: 2449–2455
Blanco-Gutierrez V, Saez-Puche R, Torralvo-Fernandez MJ. Superparamagnetism and interparticle interactions in ZnFe2O4 nanocrystals. J Mater Chem, 2012, 22: 2992
Singh JP, Kaur B, Sharma A, et al. Mechanistic insights into the interaction between energetic oxygen ions and nanosized ZnFe2O4: XAS-XMCD investigations. Phys Chem Chem Phys, 2018, 20: 12084–12096
Rodríguez Torres CE, Golmar F, Ziese M, et al. Evidence of defect-induced ferromagnetism in ZnFe2O4 thin films. Phys Rev B, 2011, 84: 064404
Salcedo Rodríguez KL, Stewart SJ, Mendoza Zélis PM, et al. Role of defects on the magnetic behaviour of the geometrically frustrated spinel ZnFe2O4. J Alloys Compd, 2018, 752: 289–295
Zviagin V, Kumar Y, Lorite I, et al. Ellipsometric investigation of ZnFe2O4 thin films in relation to magnetic properties. Appl Phys Lett, 2016, 108: 131901
Sahu BN, Suresh KG, Venkataramani N, et al. Temperature and field dependent magnetization studies on nano-crystalline ZnFe2O4 thin films. AIP Adv, 2018, 8: 056118
Sapna #, Budhiraja N, Kumar V, et al. Shape-controlled synthesis of superparamagnetic ZnFe2O4 hierarchical structures and their comparative structural, optical and magnetic properties. Ceramics Int, 2019, 45: 1067–1076
Zhao H, Liu R, Zhang Q, et al. Effect of surfactant amount on the morphology and magnetic properties of monodisperse ZnFe2O4 nanoparticles. Mater Res Bull, 2016, 75: 172–177
Xue H, Li Z, Wang X, et al. Facile synthesis of nanocrystalline zinc ferrite via a self-propagating combustion method. Mater Lett, 2007, 61: 347–350
Han JK, Choi HJ. Non-stoichiometric zinc-doped spinel ferrite nanoparticles with enhanced magnetic property and their magnetorheology. Colloid Polym Sci, 2018, 296: 405–409
Seetha Rama Raju V. Synthesis of non-stoichiometric zinc ferrite for electromagnetic wave absorber applications. Mater Sci Eng-B, 2017, 224: 88–92
Venkateshvaran D, Althammer M, Nielsen A, et al. Epitaxial ZnxFe3xO4 thin films: A spintronic material with tunable electrical and magnetic properties. Phys Rev B, 2009, 79: 134405
Makovec D, Drofenik M. Non-stoichiometric zinc-ferrite spinel nanoparticles. J Nanopart Res, 2008, 10: 131–141
Gao XC, Ma XX, Kang X, et al. Oxidative absorption of NO by sodium persulfate coupled with Fe2+, Fe3O4, and H2O2. Environ Prog Sustain Energy, 2015, 34: 117–124
Motaung DE, Mhlongo GH, Nkosi SS, et al. Shape-selective dependence of room temperature ferromagnetism induced by hierarchical ZnO nanostructures. ACS Appl Mater Interfaces, 2014, 6: 8981–8995
Sun Y, Zong Y, Feng J, et al. Oxygen vacancies driven size-dependent d0 room temperature ferromagnetism in well-dispersed dopant-free ZnO nanoparticles and density functional theory calculation. J Alloys Compd, 2018, 739: 1080–1088
Topkaya R, Baykal A, Demir A. Yafet-Kittel-type magnetic order in Zn-substituted cobalt ferrite nanoparticles with uniaxial anisotropy. J Nanopart Res, 2013, 15: 1359
Zeng X, Zhang J, Zhu S, et al. Direct observation of cation distributions of ideal inverse spinel CoFe2O4 nanofibres and correlated magnetic properties. Nanoscale, 2017, 9: 7493–7500
Lacorre P, Pannetier J, Pebler J, et al. Ordered magnetic frustration: XVII. is BaMnFeF7 frustrated? Mössbauer spectroscopy, magnetic susceptibility, and magnetic structure at 2 K. J Solid State Chem, 1992, 101: 296–308
Felner I, Yaron U, Nowik I, et al. New magnetic phases in the La-Sr-Cu-O system. Physica C-Supercond, 1992, 198: 14–18
Bedanta S, Kleemann W. Supermagnetism. J Phys D-Appl Phys, 2009, 42: 013001
Pérez N, Bartolomé F, García LM, et al. Nanostructural origin of the spin and orbital contribution to the magnetic moment in Fe3−xO4 magnetite nanoparticles. Appl Phys Lett, 2009, 94: 093108
Krishnan KM. Fundamentals and Applications of Magnetic Materials. Seattle: Oxford University Press, 2016
Zhang W, Wong PKJ, Zhang D, et al. XMCD and XMCD-PEEM studies on magnetic-field-assisted self-assembled 1D nanochains of spherical ferrite particles. Adv Funct Mater, 2017, 27: 1701265
Antonov VN, Harmon BN, Yaresko AN. Electronic structure and X-ray magnetic circular dichroism in Fe3O4 and Mn-, Co-, or Ni-substituted Fe3O4. Phys Rev B, 2003, 67: 024417
Genuzio F, Menteş TO, Freindl K, et al. Chemistry-dependent magnetic properties at the FeNi oxide-metal interface. J Mater Chem C, 2020, 8: 5777–5785
Zhu Y, Zhan Q, Yang JC, et al. Enhanced structural and magnetic coupling in a mesocrystal-assisted nanocomposite. ACS Appl Mater Interfaces, 2016, 8: 1104–1111
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51572218, 11504293 and 11904275), the Natural Science Foundation of Shaanxi Province (2019JM-138), the Scientific Research Program Funded by Shaanxi Provincial Education Department (18JK0786, 19JK0413 and 20JK0946), and the Key Project of Research and Development of Shaanxi Province (2018ZDCXL-GY-08-05). The staff of Singapore Synchrotron Light Source and beam time from SINS beamline were acknowledged for their assistance.
Author information
Authors and Affiliations
Contributions
Author contributions Sun Y performed the experiments and theoretical calculation; Deng X conducted the STEM characterization and QSTEM analysis; Li X, Peng Y, and Zheng X designed the project; Chi X carried out the XMCD characterization and analysis; Zong Y completed the XPS characterization; Deng X and Zhang J performed the EELS analysis; Feng J performed the TEM characterization; Shi Z performed the magnetic analysis; Sun Y, Li X, and Zheng X wrote the draft of manuscript; Li X and Zheng X revised the manuscript. All authors contributed to the general discussion and checked the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Yong Sun received his BSc degree from Northwest University in 2011. He is currently a PhD candidate at the School of Physics, Northwest University. He was an exchange student of Nanyang Technological University (NTU) in Singapore (November 2019-April 2020). His research focuses on magnetic materials and microwave absorption materials.
** magneto-related instruments and in situ electron microscopes and characterizing the microstructures and dynamical behaviors of low-dimensional magnetic materials and devices.
Electronic supplementary material
40843_2020_1592_MOESM1_ESM.pdf
Cation ratio and oxygen defects for engineering the magnetic transition of monodisperse nonstoichiometric zinc ferrite nanoparticles
Rights and permissions
About this article
Cite this article
Sun, Y., Deng, X., Zong, Y. et al. Cation ratio and oxygen defects for engineering the magnetic transition of monodisperse nonstoichiometric zinc ferrite nanoparticles. Sci. China Mater. 64, 2017–2028 (2021). https://doi.org/10.1007/s40843-020-1592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1592-y