Abstract
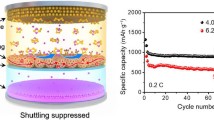
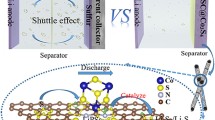
The polysulfide shuttling and sluggish redox kinetics, due to the notorious adsorption-catalysis underperformance, are the ultimate obstacles of the practical application of lithium-sulfur (Li-S) batteries. Conventional carbon-based and transition metal compound-based material solutions generally suffer from poor catalysis and adsorption, respectively, despite the performance gain in terms of the other. Herein, we have enhanced polysulfide adsorption-catalytic capability and protected the Li anode using a complementary bimetallic carbide electrocatalyst, Co3Mo3C, modified commercial separator. With this demonstration, the potentials of bimetal compounds, which have been well recognized in other environmental catalysis, are also extended to Li-S batteries. Coupled with this modified separator, a simple cathode (S/Super P composite) can deliver high sulfur utilization, high rate performance, and excellent cycle stability with a low capacity decay rate of ∼0.034% per cycle at 1 C up to 1000 cycles. Even at a high S-loading of 8.0 mg cm−2 with electrolyte/sulfur ratio=6 mL g−1, the cathode still exhibits high areal capacity of ∼6.8 mA h cm−2. The experimental analysis and the first principles calculations proved that the bimetallic carbide Co3Mo3C provides more binding sites for adsorbing polysulfides and catalyzing the multiphase conversion of sulfur/polysulfide/sulfide than monometallic carbide Mo2C. Moreover, the modified separator can be reutilized with comparable electrochemical performance. We also showed other bimetallic carbides with similar catalytic effects on Li S batteries and this material family has great promise in different energy electrocatalytic systems.
摘要
本文报道了一种钴-钼双金属碳化物(Co3Mo3C)催化材料用于修饰锂硫电池隔膜, **化多硫化锂的化学吸附和催化转化. 所组 装的电池表现出优异的电化学性能, 即使在8.0 mg cm−2的硫面积负载量条件下, 面积比容量仍高达6.8 mA h cm−2. 理论计算结果表明, 相比于单一金属碳化物Mo2C, 双金属碳化物Co3Mo3C具有更多的活性位点, 更利于化学固定多硫化锂, 并催化多硫化锂间相互转化; 同时, Ni3Mo3C和Fe3Mo3C亦表现出类似的高催化活性. 本研究对高性能锂硫电池关键催化材料的设计具有一定的指导意义.
Similar content being viewed by others
References
Griffith KJ, Wiaderek KM, Cibin G, et al. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature, 2018, 559: 556–563
Choi JW, Aurbach D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater, 2016, 1: 16013
Liu Y, Zhu Y, Cui Y. Challenges and opportunities towards fast-charging battery materials. Nat Energy, 2019, 4: 540–550
Pang Q, Liang X, Kwok CY, et al. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat Energy, 2016, 1: 16132
Manthiram A, Fu Y, Chung SH, et al. Rechargeable lithium-sulfur batteries. Chem Rev, 2014, 114: 11751–11787
Li G, Wang S, Zhang Y, et al. Revisiting the role of polysulfides in lithium-sulfur batteries. Adv Mater, 2018, 30: 1705590
Betz J, Bieker G, Meister P, et al. Theoretical versus practical energy: A plea for more transparency in the energy calculation of different rechargeable battery systems. Adv Energy Mater, 2019, 9: 1803170
Peng HJ, Huang JQ, Cheng XB, et al. Review on high-loading and high-energy lithium-sulfur batteries. Adv Energy Mater, 2017, 7: 1700260
Fang R, Zhao S, Sun Z, et al. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv Mater, 2017, 29: 1606823
Lin D, Liu Y, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat Nanotech, 2017, 12: 194–206
Li G, Gao Y, He X, et al. Organosulfide-plasticized solid-electrolyte interphase layer enables stable lithium metal anodes for long-cycle lithium-sulfur batteries. Nat Commun, 2017, 8: 850
Dai H, Gu X, Dong J, et al. Stabilizing lithium metal anode by octaphenyl polyoxyethylene-lithium complexation. Nat Commun, 2020, 11: 643
**ang Y, Li J, Lei J, et al. Advanced separators for lithium-ion and lithium-sulfur batteries: A review of recent progress. ChemSusChem, 2016, 9: 3023–3039
Xu N, Qian T, Liu X, et al. Greatly suppressed shuttle effect for improved lithium sulfur battery performance through short chain intermediates. Nano Lett, 2017, 17: 538–543
Zhang L, Ling M, Feng J, et al. The synergetic interaction between LiNO3 and lithium polysulfides for suppressing shuttle effect of lithium-sulfur batteries. Energy Storage Mater, 2018, 11: 24–29
Huang JQ, Zhang Q, Wei F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries: Progress and prospects. Energy Storage Mater, 2015, 1: 127–145
Bai S, Liu X, Zhu K, et al. Metal-organic framework-based separator for lithium-sulfur batteries. Nat Energy, 2016, 1: 16094
Jeong YC, Kim JH, Nam S, et al. Rational design of nanostructured functional interlayer/separator for advanced Li-S batteries. Adv Funct Mater, 2018, 18: 1707411
Yuan H, Peng HJ, Li BQ, et al. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithiumsulfur batteries. Adv Energy Mater, 2019, 9: 1802768
Fan L, Li M, Li X, et al. Interlayer material selection for lithium-sulfur batteries. Joule, 2019, 3: 361–386
Kong L, ** Q, Zhang XT, et al. Towards full demonstration of high areal loading sulfur cathode in lithium-sulfur batteries. J Energy Chem, 2019, 39: 17–22
Li BQ, Peng HJ, Chen X, et al. Polysulfide electrocatalysis on framework porphyrin in high-capacity and high-stable lithium-sulfur batteries. CCS Chem, 2019,: 128–137
Yao H, Yan K, Li W, et al. Improved lithium-sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode-separator interface. Energy Environ Sci, 2014, 7: 3381–3390
Chung SH, Manthiram A. High-performance Li-S batteries with an ultra-lightweight MWCNT-coated separator. J Phys Chem Lett, 2014, 5: 1978–1983
Peng HJ, Wang DW, Huang JQ, et al. Janus separator of polypropylene-supported cellular graphene framework for sulfur cathodes with high utilization in lithium-sulfur batteries. Adv Sci, 2016, 3: 1500268
Lei T, Chen W, Lv W, et al. Inhibiting polysulfide shuttling with a graphene composite separator for highly robust lithium-sulfur batteries. Joule, 2018, 2: 2091–2104
Pang Y, Wei J, Wang Y, et al. Synergetic protective effect of the ultralight MWCNTs/NCQDs modified separator for highly stable lithium-sulfur batteries. Adv Energy Mater, 2018, 8: 1702288
Fu A, Wang C, Pei F, et al. Recent advances in hollow porous carbon materials for lithium-sulfur batteries. Small, 2019, 15: 1804786
Guo Z, Zhang B, Li D, et al. A mixed microporous/low-range mesoporous composite with high sulfur loading from hierarchically-structured carbon for lithium sulfur batteries. Electrochim Acta, 2017, 230: 181–188
Kang N, Lin Y, Yang L, et al. Cathode porosity is a missing key parameter to optimize lithium-sulfur battery energy density. Nat Commun, 2019, 10: 4597
Peng HJ, Zhang ZW, Huang JQ, et al. A cooperative interface for highly efficient lithium-sulfur batteries. Adv Mater, 2016, 28: 9551–9558
Seh ZW, Yu JH, Li W, et al. Two-dimensional layered transition metal disulphides for effective encapsulation of high-capacity lithium sulphide cathodes. Nat Commun, 2014, 5: 5017
Ghazi ZA, He X, Khattak AM, et al. MoS2/celgard separator as efficient polysulfide barrier for long-life lithium-sulfur batteries. Adv Mater, 2017, 29: 1606817
Zhao P, Zhang Z, He H, et al. Cobalt-tungsten bimetallic carbide nanoparticles as efficient catalytic material for high-performance lithium-sulfur batteries. ChemSusChem, 2019, 12: 4866–4873
Cheng Z, Pan H, Chen J, et al. Separator modified by cobalt-embedded carbon nanosheets enabling chemisorption and catalytic effects of polysulfides for high-energy-density lithium-sulfur batteries. Adv Energy Mater, 2019, 9: 1901609
Tao X, Wang J, Liu C, et al. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design. Nat Commun, 2016, 7: 11203
Zhang J, Li Z, Chen Y, et al. Nickel-iron layered double hydroxide hollow polyhedrons as a superior sulfur host for lithium-sulfur batteries. Angew Chem Int Ed, 2018, 57: 10944–10948
Shao AH, Zhang Z, **ong DG, et al. Facile synthesis of a “two-in-one” sulfur host featuring metallic-cobalt-embedded N-doped carbon nanotubes for efficient lithium-sulfur batteries. ACS Appl Mater Interfaces, 2020, 12: 5968–5978
Huang M, Yang J, ** B, et al. Enhancing kinetics of Li-S batteries by graphene-like N,S-codoped biochar fabricated in NaCl nonaqueous ionic liquid. Sci China Mater, 2019, 62: 455–464
Wang Y, Zhang R, Chen J, et al. Enhancing catalytic activity of titanium oxide in lithium-sulfur batteries by band engineering. Adv Energy Mater, 2019, 9: 1900953
Zhang Z, Shao AH, **ong DG, et al. Efficient polysulfide redox enabled by lattice-distorted Ni3Fe intermetallic electrocatalyst-modified separator for lithium-sulfur batteries. ACS Appl Mater Interfaces, 2020, 12: 19572–19580
Ye H, Sun J, Zhang S, et al. Stepwise electrocatalysis as a strategy against polysulfide shuttling in Li-S batteries. ACS Nano, 2019, 13: 14208–14216
Zhao M, Peng HJ, Zhang ZW, et al. Activating inert metallic compounds for high-rate lithium-sulfur batteries through in situ etching of extrinsic metal. Angew Chem Int Ed, 2019, 58: 3779–3783
Du Z, Chen X, Hu W, et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J Am Chem Soc, 2019, 141: 3977–3985
Zhao CX, Li BQ, Zhao M, et al. Precise anionic regulation of NiFe hydroxysulfide assisted by electrochemical reactions for efficient electrocatalysis. Energy Environ Sci, 2020, 13: 1711–1716
Alonso DM, Wettstein SG, Dumesic JA. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem Soc Rev, 2012, 41: 8075–8098
Sankar M, Dimitratos N, Miedziak PJ, et al. Designing bimetallic catalysts for a green and sustainable future. Chem Soc Rev, 2012, 41: 8099–8139
Luo M, Zhao Z, Zhang Y, et al. PdMo bimetallene for oxygen reduction catalysis. Nature, 2019, 574: 81–85
Zhang Z, Wu DH, Zhou Z, et al. Sulfur/nickel ferrite composite as cathode with high-volumetric-capacity for lithium-sulfur battery. Sci China Mater, 2019, 62: 74–86
Puello-Polo E, Brito JL. Effect of the type of precursor and the synthesis method on thiophene hydrodesulfurization activity of activated carbon supported Fe-Mo, Co-Mo and Ni-Mo carbides. J Mol Catal A-Chem, 2008, 281: 85–92
Guo L, Wang J, Teng X, et al. A novel bimetallic nickel-molybdenum carbide nanowire array for efficient hydrogen evolution. ChemSusChem, 2018, 11: 2717–2723
Liu Y, Li GD, Yuan L, et al. Carbon-protected bimetallic carbide nanoparticles for a highly efficient alkaline hydrogen evolution reaction. Nanoscale, 2015, 7: 3130–3136
Zhang Z, Kong LL, Liu S, et al. A high-efficiency sulfur/carbon composite based on 3D graphene nanosheet@carbon nanotube matrix as cathode for lithium-sulfur battery. Adv Energy Mater, 2017, 7: 1602543
Chen T, Zhang Z, Cheng B, et al. Self-templated formation of interlaced carbon nanotubes threaded hollow Co3S4 nanoboxes for high-rate and heat-resistant lithium-sulfur batteries. J Am Chem Soc, 2017, 139: 12710–12715
Mi Y, Liu W, Li X, et al. High-performance Li-S battery cathode with catalyst-like carbon nanotube-MoP promoting polysulfide redox. Nano Res, 2017, 10: 3698–3705
Zeng X, Gao X, Li G, et al. Conductive molybdenum carbide as the polysulfide reservoir for lithium-sulfur batteries. J Mater Chem A, 2018, 6: 17142–17147
Liang X, Kwok CY, Lodi-Marzano F, et al. Tuning transition metal oxide-sulfur interactions for long life lithium sulfur batteries: The “goldilocks” principle. Adv Energy Mater, 2016, 6: 1501636
Yin LC, Liang J, Zhou GM, et al. Understanding the interactions between lithium polysulfides and N-doped graphene using density functional theory calculations. Nano Energy, 2016, 25: 203–210
Su D, Cortie M, Wang G. Fabrication of N-doped graphene-carbon nanotube hybrids from Prussian blue for lithium-sulfur batteries. Adv Energy Mater, 2017, 7: 1602014
Xu ZL, Lin S, Onofrio N, et al. Exceptional catalytic effects of black phosphorus quantum dots in shuttling-free lithium sulfur batteries. Nat Commun, 2018, 9: 4164
** K, Chen B, Li H, et al. Soluble polysulphide sorption using carbon nanotube forest for enhancing cycle performance in a lithium-sulphur battery. Nano Energy, 2015, 12: 538–546
** K, He D, Harris C, et al. Enhanced sulfur transformation by multifunctional FeS2/FeS/S composites for high-volumetric capacity cathodes in lithium-sulfur batteries. Adv Sci, 2019, 6: 1800815
Zhang Z, Basu S, Zhu P, et al. Highly sulfiphilic Ni-Fe bimetallic oxide nanoparticles anchored on carbon nanotubes enable effective immobilization and conversion of polysulfides for stable lithium-sulfur batteries. Carbon, 2019, 142: 32–39
Dai H, ** K, Liu X, et al. Cationic surfactant-based electrolyte additives for uniform lithium deposition via lithiophobic repulsion mechanisms. J Am Chem Soc, 2018, 140: 17515–17521
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21863006, 51662029, 61974082 and 61704096), Youth Science Foundation of Jiangxi Province (20192BAB216001), and Key Laboratory of Jiangxi Province for Environment and Energy Catalysis (20181BCD40004).
Author information
Authors and Affiliations
Contributions
Author contributions Zhang Z, Yang ZY and ** K conceived the idea; Zhang Z prepared the materials, conducted the electrochemical experiments; Shao AH and **ong DG conducted the characterization of materials; Li HL performed the DFT calculations; Wang JN, Liu JW, Lao CY, Lu SY, Jiang Q, Yu J, Li HL and Kumar RV contributed to the correction of the manuscript; ** K and Yang ZY revised the manuscript written by Zhang Z. All the authors commented on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Ze Zhang is a lecturer at the College of Chemistry, Nanchang University. He received his BSc degree in 2012 from the College of Chemistry, and PhD degree in 2017 from the School of Materials Science and Engineering, Nankai University, China. His main research interest is in the area of advanced functional materials for rechargeable batteries with a focus on the exploration of high-energy Li-S batteries.
Kai ** is a research associate at the Cambridge Graphene Centre. He obtained his PhD degree in the Department of Materials Science and Metallurgy, University of Cambridge. His research focuses on materials engineering and physical chemistry for renewable energy conversion and storage, in particular lithium-sulphur (Li-S) batteries, lithium-ion batteries and solar cells. He was awarded the grand prize of the Dow Sustainability Innovation Student Challenge Award. His CamBattery team was awarded Technology Start Up of the Year by Cambridge University Entrepreneurs in 2012.
Huang-Long Li is an associate professor at the Department of Precision Instrument, Tsinghua University. His research interests include nanoelectronics, neuromorphic engineering, ab initio calculations, electronic materials and energy materials. He received his PhD degree in electrical engineering from the University of Cambridge (2014), and the BSc degree in physics from Peking University (2010).
Zhen-Yu Yang is a professor at the School of Chemistry, Nanchang University, China. He received his PhD degree at the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences in 2005. He used to work as a visiting research fellow at Rensselaer Polytechnic Institute in USA from 2012 to 2013 and at Nanyang Technological University in Singapore in 2019, respectively. Currently, his main research focuses on energy storage materials for power sources, including Li-ion batteries, Li-S batteries, and supercapacitors.
Supporting Information
40843_2020_1425_MOESM1_ESM.pdf
Recyclable cobalt-molybdenum bimetallic carbide modified separator boosts the polysulfide adsorption-catalysis of lithium sulfur battery
Rights and permissions
About this article
Cite this article
Zhang, Z., Wang, JN., Shao, AH. et al. Recyclable cobalt-molybdenum bimetallic carbide modified separator boosts the polysulfide adsorption-catalysis of lithium sulfur battery. Sci. China Mater. 63, 2443–2455 (2020). https://doi.org/10.1007/s40843-020-1425-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1425-2