Abstract
Background
Menopause, a dramatical estrogen-deficient condition, is considered the most significant milestone in women’s health.
Purpose
To investigate the metabolite changes attributed to estrogen deficiency using random forest (RF)-based machine learning (ML) modeling strategy in ovariectomized (OVX) mice as well as determine the clinical relevance of selected metabolites in older women.
Methods and results
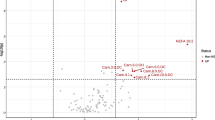
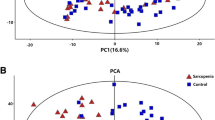
Untargeted and targeted metabolomic analyses revealed that metabolites related to TCA cycle, sphingolipids, phospholipids, fatty acids, and amino acids, were significantly changed in the plasma and/or muscle of OVX mice. Subsequent ML classifiers based on RF algorithm selected alpha-ketoglutarate (AKG), arginine, carnosine, ceramide C24, phosphatidylcholine (PC) aa C36:6, and PC ae C42:3 in plasma as well as PC aa 34:1, PC aa C34:3, PC aa C36:5, PC aa C32:1, PC aa C36:2, and sphingosine in muscle as top featured metabolites that differentiate the OVX mice from the sham-operated group. When circulating levels of AKG, arginine, and carnosine, which showed the most significant changes in OVX mice blood, were measured in postmenopausal women, higher plasma AKG levels were associated with lower bone mass, weak grip strength, poor physical performance, and increased frailty risk.
Conclusions
Metabolomics- and ML-based methods identified the key metabolites of blood and muscle that were significantly changed after ovariectomy in mice, and the clinical implication of several metabolites was investigated by looking at their correlation with body composition and frailty-related parameters in postmenopausal women. These findings provide crucial context for understanding the diverse physiological alterations caused by estrogen deficiency in women.






Similar content being viewed by others
Data availability
Some or all datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Eyster KM (2016) The estrogen receptors: an overview from different perspectives. Methods Mol Biol 1366:1–10
Hamilton KJ, Hewitt SC, Arao Y, Korach KS (2017) Estrogen hormone biology. Curr Top Dev Biol 125:109–146
Lizcano F (2022) Roles of estrogens, estrogen-like compounds, and endocrine disruptors in adipocytes. Front Endocrinol (Lausanne) 13:921504
Bracht JR, Vieira-Potter VJ, De Souza SR, Öz OK, Palmer BF, Clegg DJ (2020) The role of estrogens in the adipose tissue milieu. Ann N Y Acad Sci 1461:127–143
Patel S, Homaei A, Raju AB, Meher BR (2018) Estrogen: the necessary evil for human health, and ways to tame it. Biomed Pharmacother 102:403–411
Nelson HD (2008) Menopause. Lancet 371:760–770
Santoro N, Roeca C, Peters BA, Neal-Perry G (2021) The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab 106:1–15
Geraci A, Calvani R, Ferri E, Marzetti E, Arosio B, Cesari M (2021) Sarcopenia and menopause: the role of estradiol. Front Endocrinol (Lausanne) 12:682012
Ruan H, Hu J, Zhao J, Tao H, Chi J, Niu X et al (2020) Menopause and frailty: a sco** review. Menopause 27:1185–1195
Sophocleous A, Idris AI (2014) Rodent models of osteoporosis. BoneKEy Rep 3:614–614
Sun B, Yin YZ, **ao J (2019) An in vivo estrogen deficiency mouse model for screening exogenous estrogen treatments of cardiovascular dysfunction after menopause. J Vis Exp. https://doi.org/10.3791/59536-v
Tao X, Yan M, Wang L, Zhou Y, Wang Z, **a T et al (2020) Effects of estrogen deprivation on memory and expression of related proteins in ovariectomized mice. Ann Transl Med 8:356
Zhao H, Li X, Zhang D, Chen H, Chao Y, Wu K et al (2018) Integrative bone metabolomics-lipidomics strategy for pathological mechanism of postmenopausal osteoporosis mouse model. Sci Rep 8:16456
Souza VR, Mendes E, Casaro M, Antiorio A, Oliveira FA, Ferreira CM (2019) Description of ovariectomy protocol in mice. Methods Mol Biol 1916:303–309
Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradère JP, Le Gonidec S et al (2018) The exerkine apelin reverses age-associated sarcopenia. Nat Med 24:1360–1371
Lee SW, Yang J, Kim SY, Jeong HK, Lee J, Kim WJ et al (2015) MicroRNA-26a induced by hypoxia targets HDAC6 in myogenic differentiation of embryonic stem cells. Nucleic Acids Res 43:2057–2073
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Kim HS, Yoo HJ, Lee KM, Song HE, Kim SJ, Lee JO et al (2021) Stearic acid attenuates profibrotic signalling in idiopathic pulmonary fibrosis. Respirology 26:255–263
Kim HK, Shin MS, Youn BS, Kang GM, Gil SY, Lee CH et al (2015) Regulation of energy balance by the hypothalamic lipoprotein lipase regulator Angptl3. Diabetes 64:1142–1153
Koh EH, Yoon JE, Ko MS, Leem J, Yun JY, Hong CH et al (2021) Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut 70:1954–1964
Lee SR, Heo JH, Jo SL, Kim G, Kim SJ, Yoo HJ et al (2021) Progesterone receptor membrane component 1 reduces cardiac steatosis and lipotoxicity via activation of fatty acid oxidation and mitochondrial respiration. Sci Rep 11:8781
Lee HY, Nam S, Kim MJ, Kim SJ, Back SH, Yoo HJ (2021) Butyrate prevents TGF-β1-induced alveolar myofibroblast differentiation and modulates energy metabolism. Metabolites 11:258
Ji M, Jo Y, Choi SJ, Kim SM, Kim KK, Oh B-C et al (2022) Plasma metabolomics and machine learning-driven novel diagnostic signature for non-alcoholic steatohepatitis. Biomedicines 10:1669
Oh J-H, Song S, Rhee H, Lee SH, Kim DY, Choe JC et al (2019) Normal reference plots for the bioelectrical impedance vector in healthy Korean adults. J Korean Med Sci 34:e198
Nga HT, Jang IY, Kim DA, Park SJ, Lee JY, Lee S et al (2021) Serum GDF15 level is independent of sarcopenia in older Asian adults. Gerontology 67:525–531
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C et al (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429
Jung HW, Park JH, Kim DA, Jang IY, Park SJ, Lee JY et al (2021) Association between serum FGF21 level and sarcopenia in older adults. Bone 145:115877
Kim DH, Afilalo J, Shi SM, Popma JJ, Khabbaz KR, Laham RJ et al (2019) Evaluation of changes in functional status in the year after aortic valve replacement. JAMA Intern Med 179:383–391
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Series A 56:M146–M157
Jang IY, Lee S, Kim JH, Lee E, Lee JY, Park SJ et al (2020) Lack of association between circulating apelin level and frailty-related functional parameters in older adults: a cross-sectional study. BMC Geriatr 20:420
Rockwood K, Mitnitski A (2007) Frailty in relation to the accumulation of deficits. J Gerontol Series A 62:722–727
Lee H, Lee E, Jang I-Y (2020) Frailty and comprehensive geriatric assessment. J Korean Med Sci. https://doi.org/10.3346/jkms.2020.35.e16
Kim SJ, Song HE, Lee HY, Yoo HJ (2021) Mass spectrometry-based metabolomics in translational research. Adv Exp Med Biol 1310:509–531
Schooneman MG, Vaz FM, Houten SM, Soeters MR (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62:1–8
Biolo G, Cederholm T, Muscaritoli M (2014) Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr 33:737–748
Karakelides H, Nair KS (2005) Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol 68:123–148
Wilkinson DJ, Rodriguez-Blanco G, Dunn WB, Phillips BE, Williams JP, Greenhaff PL et al (2020) Untargeted metabolomics for uncovering biological markers of human skeletal muscle ageing. Aging (Albany NY) 12:12517–12533
Uchitomi R, Hatazawa Y, Senoo N, Yoshioka K, Fujita M, Shimizu T et al (2019) Metabolomic analysis of skeletal muscle in aged mice. Sci Rep 9:10425
Moaddel R, Fabbri E, Khadeer MA, Carlson OD, Gonzalez-Freire M, Zhang P et al (2016) Plasma biomarkers of poor muscle quality in older men and women from the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci 71:1266–1272
Peterson CM, Johannsen DL, Ravussin E (2012) Skeletal muscle mitochondria and aging: a review. J Aging Res 2012:194821
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S et al (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 102:5618–5623
Su Y, Wang T, Wu N, Li D, Fan X, Xu Z et al (2019) Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging (Albany NY) 11:4183–4197
Asadi Shahmirzadi A, Edgar D, Liao CY, Hsu YM, Lucanic M, Asadi Shahmirzadi A et al (2020) Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab 32:447-456.e446
Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D (2016) Alpha-ketoglutarate: physiological functions and applications. Biomol Ther (Seoul) 24:1–8
Cai X, Yuan Y, Liao Z, **ng K, Zhu C, Xu Y et al (2018) α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway. Faseb j 32:488–499
Tomaszewska E, Świątkiewicz S, Arczewska-Włosek A, Wojtysiak D, Dobrowolski P, Domaradzki P et al (2020) Alpha-ketoglutarate: an effective feed supplement in improving bone metabolism and muscle quality of laying hens: a preliminary study. Animals (Basel) 10:2420
Sutham W, Sripetchwandee J, Minta W, Mantor D, Pattanakuhar S, Palee S et al (2018) Ovariectomy and obesity have equal impact in causing mitochondrial dysfunction and impaired skeletal muscle contraction in rats. Menopause 25:1448–1458
McCoin CS, Knotts TA, Adams SH (2015) Acylcarnitines–old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol 11:617–625
Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A et al (2004) Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab 89:3864–3871
Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA (2010) The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci 65:119–128
Barbul A (2008) Proline precursors to sustain Mammalian collagen synthesis. J Nutr 138:2021s–2024s
Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003) Skin aging and menopause: implications for treatment. Am J Clin Dermatol 4:371–378
Funding
This study was supported by grants from the National Research Foundation of Korea (NRF) funded by the South Korean government (MSIT) [grant number: 2021R1C1C2006842] and from the Asan Institute for Life Science, Asan Medical Center, Seoul, South Korea [grant number: 2022IP0036].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no present or potential conflicts of interest.
Ethical approval
The present study received the approval of the AMC Institutional Review Board (no. 2020–0259).
Informed consent
Informed consent was obtained from each study participant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S.J., Jo, Y., Park, S.J. et al. Metabolomic profiles of ovariectomized mice and their associations with body composition and frailty-related parameters in postmenopausal women. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02338-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02338-x