Abstract
Background
Nursing home (NH) residents suffered the greatest impact of the COVID-19 pandemic. Limited data are available on vaccine-induced immunity and on the protection ensured by a prior infection in this population.
Aims
The present study aims to monitor antibody levels and their persistence over a 6-month period in NH residents according to the history of prior SARS-CoV-2 infection.
Methods
We measured anti-trimeric Spike IgG antibody levels in a sample of 395 residents from 25 NHs in 6 Italian Regions at study enrolment (prior to the first dose of vaccine, T0) and then after 2 (T1) and 6 months (T2) following the first vaccine dose. All participants received mRNA vaccines (BNT162b2 or mRNA-1273). Analyses were performed using log-transformed values of antibody concentrations and geometric means (GM) were calculated.
Results
Superior humoral immunity was induced in NH residents with previous SARS-CoV-2 infection. (T0: GM 186.6 vs. 6.1 BAU/ml, p < 0.001; T1: GM 5264.1 vs. 944.4 BAU/ml, p < 0.001; T2: GM 1473.6 vs. 128.7 BAU/ml, p < 0.001). Residents with prior SARS-CoV-2 infection receiving two vaccine doses presented significantly higher antibody concentration at T1 and T2. A longer interval between previous infection and vaccination was associated with a better antibody response over time.
Discussion
In a frail sample of NH residents, prior SARS-CoV-2 infection was associated with a higher humoral response to vaccination. Number of vaccine doses and the interval between infection and vaccination are relevant parameters in determining humoral immunity.
Conclusions
These findings provide important information to plan future immunization policies and disease prevention strategies in a highly vulnerable population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus Disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has determined a substantial increment in morbidity and mortality worldwide [1]. Nursing home (NH) residents, that often present with a high burden of comorbidities and clinical complexities, suffered the greatest impact of the pandemic in many countries worldwide, independently of health care system organization [2,3,4,5]. Epidemiological data indicate that during the first pandemic wave, up to 50% of deaths from COVID-19 may have occurred within NH facilities [6]. For this reason, in many Western countries, priority has been given to this population to receive anti-SARS-CoV-2 vaccination. Starting from the end of December 2020, the Italian government has implemented vaccination programmes in NH to reduce the risk of COVID-19-related morbidity and mortality in this high-risk population [7]. Priority was given to the use of mRNA vaccines (mRNA-1273 or BNT162b2) in this population. Residents with no prior history of SARS-CoV-2 infection received two doses of mRNA vaccine. Vaccination was also recommended for residents with a history of prior SARS-CoV-2 infection. The Italian Ministry of Health recommended a single dose for those with a history of SARS-CoV-2 infection between 3 and 6 months beforehand and two doses for those with a history of infection more than 6 months beforehand [7].
Recent data indicate that immune response to vaccination tends to reduce over time and that this reduction is associated with breakthrough SARS-CoV-2 infections [8,9,10]. In addition, in healthy individuals, a prior SARS-CoV-2 infection was associated with a higher antibody level, suggesting that prior infection history may increase protection from vaccination and that different strategies and timing could be employed in the vaccination strategy, as well as in the administration of booster doses, in relation to the history of previous infection [11]. However, the available evidence cannot be generalized to NH residents. Limited data are available on the duration of the protection of vaccination and on the immunisation, coverage ensured by a prior infection in this population. In addition, immune response to vaccination might be reduced in frail NH residents with clinical complexity and it might be hypothesized that the potential benefits of hybrid immunity may also be decreased in this population compared to healthy individuals.
For these reasons, the present study aims to monitor antibody levels and their persistence over a 6-months period in NH residents according to the history of prior SARS-CoV-2 infection. This study is performed in the context of the GeroCovid Vax study [12], a multicentre Italian project monitoring effectiveness of SARS-CoV-2 vaccination in NH nationwide.
Methods
GeroCovid Vax study
GeroCOVID VAX study is promoted by the Italian Society of Gerontology and Geriatrics (SIGG) (Florence, Italy) and the Istituto Superiore di Sanità (ISS, Rome, Italy) and sponsored by the Italian Medicines Agency (AIFA). The study began in February 2021 and aimed at investigating the effects of anti-SARS-CoV-2 vaccine use in older NH residents in Italy [12]. Residents were consecutively enrolled within the vaccination calendar scheduled in each participating NH. The delay with respect to the formal start of the vaccination campaign had no impact on the representativeness of the study sample as the vaccination become fully operational in the first quarter of 2021. Main GeroCovid Vax study objectives include: (i) evaluating he efficacy and safety of the SARS-CoV-2 vaccine in a representative sample of older NH residents, and (ii) evaluate humoral and cellular immune response in a large subpopulation of study participants. The inclusion criteria were age ≥ 60 years, life expectancy ≥ 3 months, expected NH stay ≥ 3 months, receiving at least one dose of any type of anti-SARS-CoV-2 vaccine. Demographic and clinical data were collected by trained researchers at study enrolment (prior to the first dose of vaccine). These included age, sex, chronic diseases, and history of confirmed SARS-CoV-2 infection (as assessed by reverse transcriptase–polymerase chain reaction testing). Humoral immune response was evaluated in a sample of 395 residents at study enrolment (prior to the first dose of vaccine, T0) and then 2 (T1) and 6 months (T2) following the first vaccine dose.
Serum preparation and storage
All blood samples were properly prepared and stored following a standardized procedure. Blood samples were collected in Serum Separator Tubes (BD Diagnostic Systems, Franklin Lakes, NJ, USA) and centrifuged at room temperature at 1600 rpm for 10 min. Aliquots were transferred to 2 ml polypropylene screw cap cryotubes (Nunc™, Thermofisher Scientific, Waltham, MA USA) and immediately frozen at -20 °C. Frozen sera were then shipped to the ISS as a national reference laboratory for COVID-19, in dry ice following biosafety shipment condition. Upon arrival serum samples were immediately stored at − 80 °C.
SARS-CoV-2 IgG immunoassays
The Liaison® SARS-CoV-2 TrimericS IgG chemiluminescent assay (DiaSorin, Italy), using the trimeric S antigen stabilized in its native form and designed for high throughput in healthcare settings was used. The LIAISON® XL fully automated chemiluminescence analyzer automatically calculates SARS-CoV-2 trimeric S IgG antibody concentrations, expressed as binding antibody units (BAU/ml). The assay range is up to 2080 BAU/ml. According to the manufacturer’s instructions, values ≥ 33.8 BAU/ml were interpreted as positive. Samples that were above the upper limit of the assay were automatically diluted 1:20 and re-analysed.
Statistical analysis
Baseline characteristics according to prior SARS-CoV-2 infection were compared using analysis of variance (ANOVA) for normally distributed variables, nonparametric Kruskal–Wallis H tests for skewed variables and chi-square analyses for dichotomous variables. Given the non-normal distribution of SARS-CoV-2 trimeric S IgG antibody concentrations, analyses were performed using log-transformed values and geometric means were calculated (GM). Analysis of covariance (ANCOVA) was used to compare means of SARS-CoV-2 trimeric S IgG antibody concentrations according to prior SARS-CoV-2 infection. Among residents with prior SARS-CoV-2 infection antibody concentration according to number of doses received (1 vs. 2 doses) and time from SARS-CoV-2 infection (≤ 4 months vs. ≥ 5 months) was calculated. To avoid the confounding effect related to the number of vaccine doses received, the effect of time from SARS-CoV-2 infection on antibody concentration was analysed only in residents receiving a single vaccine dose. Variables considered for adjustment in the ANCOVA models were age, sex and those associated with prior SARS-CoV-2 infection at p ≤ 0.10 at the univariate analysis. Statistical analysis was carried out with STATA Software Version 16.1 (Stata Cooperation, College Station, TX, USA).
Ethical approval
The study was approved by the Italian National Ethical Committee with permission number 264/2021 (January 26, 2021).
Results
Study sample
The study sample consisted of 395 residents from 25 NHs in 6 Italian Regions. Table 1 shows resident’s characteristics according to prior SARS-CoV-2 infection. Mean age of the study sample was 82.4 ± 9.5 years and 68% of residents were women. Most residents received BNT162b2 vaccine (87%). Dementia was the most common condition observed (54% of residents), followed by hypertension (50%), ischemic heart diseases (29%), diabetes (16%) and chronic obstructive pulmonary disease (16%). Overall, 139 residents (35.2%) had SARS-CoV-2 infection prior to study enrolment. As compared with residents with prior SARS-CoV-2 infection, those without infection were older and had a significantly higher prevalence of chronic renal failure. Among the 139 residents with prior SARS-CoV-2 infection, 118 (85%) received a single vaccine dose and 21 (15%) two doses. Among the 118 residents receiving a single dose of vaccine, 80 (67.8%) had prior infection ≤ 4 months before vaccination, 33 (28.0%) ≥ 5 months before vaccination and in 5 (4.2%) residents the information on SARS-CoV-2 infection date was not available.
SARS-CoV-2 trimeric S IgG antibody concentration and prior-SARS-CoV-2 infection
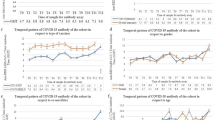
Figure 1 shows SARS-CoV-2 trimeric S IgG antibody concentration before vaccination (T0), 2 months (T1) and 6 months (T2) after the first dose according to prior SARS-CoV-2 infection. Significantly higher antibody levels were present at T0 in residents with prior SARS-CoV-2 infection. Antibody concentration reached the highest level 2 months after vaccination (T1) and then declined at 6 months (T2).
SARS-CoV-2 trimeric S IgG antibody concentration (log-transformed values) before vaccination (T0), 2 months (T1) and 6 months (T2) after first dose of vaccine according to prior SARS-CoV-2 infection. Data are presented for the whole sample (upper panel, n = 395), for BNT162b2 (n = 344, lower left panel), and mRNA-1273 vaccines (n = 51 lower right panel)
Overall, 392 (99%) residents at 2 months (T1) and 343 (87%) at 6 months after vaccination (T2) had an antibody concentration above the 33.8 BAU/ml threshold. The response to vaccination was significantly higher in residents with prior SARS-CoV-2 infection who continued to have significantly higher antibody levels at 2- (T1) and 6-month (T2) follow-ups. When data were stratified according to the type of vaccine, we found that the effect of prior SARS-CoV-2 infection on antibody concentration was consistent for both mRNA-1273 and BNT162b2 vaccines. We did not observe any significant impact of any of the conditions suffered by residents reported on the antibody response to vaccination.
Table 2 shows adjusted GM (Standard Errors) of SARS-CoV-2 trimeric S IgG antibody concentration among participating residents, according to prior SARS-CoV-2 infection. Participants with a prior infection presented a significant higher concentration of antibodies than those without a history of infection before vaccination (T0; GM 186.6 vs. 6.1 BAU/ml, p < 0.001) and at the 2- (T1; GM 5264.1 vs. 944.4 BAU/ml, p < 0.001) and 6-month (T2; GM 1473.6 vs. 128.7 BAU/ml, p < 0.001) follow-ups. This association was consistent in both users of mRNA-1273 or BNT162b2. Independently of prior SARS-CoV-2 infection, residents receiving mRNA-1273 had significantly higher antibody concentration than those receiving BNT162b2.
SARS-CoV-2 trimeric S IgG antibody concentration in residents with prior-SARS-CoV-2 infection
Table 3 shows geometric means of SARS-CoV-2 trimeric S IgG antibody concentration in residents with prior SARS-CoV-2 infection according to number of doses received and time of SARS-CoV-2 infection diagnosis. Residents with prior SARS-CoV-2 infection receiving two vaccine doses presented significantly higher antibody concentration in the 2- and 6-month follow-ups (T1 and T2). Independently of the number of doses received, residents with prior SARS-CoV-2 infection had higher antibody concentration at 2- and 6-month follow-ups than those with no SARS-CoV-2 infection history. To avoid the confounding effect related to the number of vaccine doses received, the effect of time from SARS-CoV-2 infection on antibody concentration was analysed only in residents receiving a single vaccine dose. As shown in Table 3, residents experiencing SARS-CoV-2 infection ≥ 5 months before vaccination had a lower level of antibody concentration before vaccination (T0), but this association was reversed after vaccination. At the 2- and 6-month follow-ups (T1 and T2), this group presented a significantly higher antibody concentration as compared with residents experiencing SARS-CoV-2 infection ≤ 4 months before vaccination. The multiplicative factor of a previous infection on the antibody response induced by vaccination was 11.4 at the T2 follow-up. More in detail, SARS-CoV-2 trimeric S IgG antibody concentration after a single vaccine dose, was 16.1-fold higher in residents who had the previous infection ≥ 5 months before vaccination and 7.3-fold higher in residents who had SARS-CoV-2 infection ≤ 4 months before vaccination.
Discussion
Frail NH residents suffered the most severe consequences from SARS-CoV-2 epidemic. For this reason, particular attention to the effect of prevention strategies on this population is a priority to limit the impact of the epidemic worldwide. The main finding of the present study is that significantly superior humoral immunity is induced by mRNA vaccines in NH residents with a previous SARS-CoV-2 infection compared to residents without prior infection.
Overall, the administration of SARS-CoV-2 mRNA vaccines resulted immunogenic in NH residents. Indeed, two months after the completion of the primary vaccine schedule, almost 100% of the residents showed a positive anti-trimeric Spike IgG titer. Six months after the first immunization still more than 85% of residents had a titer above the positivity cut-off of the serological assay used, an observation that extends to older adults living in NH findings results obtained in other settings and age-groups [8,9,10,11]. The number of vaccine doses influenced the antibody response. A longer interval between SARS-CoV-2 infection and vaccination was associated with a better humoral immune response.
It has been shown that in subjects previously infected by SARS-CoV-2, vaccination increased all humoral response components [13]. The potentiating effect of a previous infection is not surprising and could be attributed to the so-called hybrid immunity [14]. Immunological memory generated after exposure to SARS-CoV-2 is promptly activated by subsequent vaccine immunization, leading to an enhanced immune response [14].
The hybrid immunity effect on the magnitude of vaccine-induced humoral response has been shown in several studies. Among vaccinated health care workers from the UK, previously infected individuals expressed higher levels of anti-SARS-CoV-2 IgG than those with no previous SARS-CoV-2 infection after a single dose of BNT162b2 [ World Health Organization. Weekly epidemiological update on COVID-19. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 15 Feb 2022 Palmieri L, Vanacore N, Donfrancesco C et al (2020) Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci 75:1796–1800 Heckman GA, Kay K, Morrison A et al (2021) Proceedings from an international virtual townhall: reflecting on the COVID-19 pandemic: themes from long-term care. J Am Med Dir Assoc 22:1128–1132 Fisman DN, Bogoch I, Lapointe-Shaw L et al (2020) Risk Factors Associated With Mortality Among Residents With Coronavirus Disease 2019 (COVID-19) in Long-term Care Facilities in Ontario, Canada. JAMA Netw Open 3:e2015957 Malara A, Noale M, Abbatecola AM et al (2022) Clinical features of SARS-CoV-2 infection in italian long-term care facilities: GeroCovid LTCFs observational study. J Am Med Dir Assoc 23:15–18 Sepulveda ER, Stall NM, Sinha SK (2020) A comparison of COVID-19 mortality rates among long-term care residents in 12 OECD countries. J Am Med Dir Assoc 21:1572-1574.e3 Guidelines of the Strategic Plan on COVID-19 vaccines approved by Parliament. Recommendations for the Organization of the Vaccination Campaign against SARS-CoV-2/COVID-19 and Vaccination Procedure. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=77981&parte=1&serie=null. Accessed 24 Dec 2020 McDade TW, Demonbreun AR, Sancilio A et al (2021) Durability of antibody response to vaccination and surrogate neutralization of emerging variants based on SARS-CoV-2 exposure history. Sci Rep 11:17325 Naaber P, Tserel L, Kangro K et al (2021) Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur 10:100208 Milne G, Hames T, Scotton C et al (2021) Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med 9:1450–1466 Manisty C, Otter AD, Treibel TA et al (2021) Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 397:1057–1058 Abbatecola A, Antonelli Incalzi R, Malara A, et al (2021) Disentangling the impact of COVID-19 infection on clinical outcomes and preventive strategies in older persons: an Italian perspective. J Gerontol Geriatr 1–9 Wang Z, Muecksch F, Schaefer-Babajew D et al (2021) Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 595:426–431 Crotty S (2021) Hybrid immunity. Science 372:1392–1393 Zhong D, **ao S, Debes AK et al (2021) Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA 326:2524–2526 Abu Jabal K, Ben-Amram H, Beiruti K et al (2021) Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 26:2100096 Ebinger JE, Fert-Bober J, Printsev I et al (2021) Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 27:981–984 Martínek J, Tomášková H, Janošek J et al (2022) Immune response 5–7 months after vaccination against SARS-CoV-2 in Elderly Nursing Home Residents in the Czech Republic: Comparison of Three Vaccines. Viruses 14:1086 Jeulin H, Labat C, Duarte K et al (2022) Anti-spike IgG antibody kinetics following the second and third doses of BNT162b2 vaccine in nursing home residents. J Am Geriatr Soc. https://doi.org/10.1111/jgs.17837 Nikolich-Žugich J (2018) The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 19:10–19 Ridda I, Macintyre CR, Lindley R et al (2009) Immunological responses to pneumococcal vaccine in frail older people. Vaccine 27:1628–1636 Yao X, Hamilton RG, Weng NP et al (2011) Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 29:5015–5021 Payne RP, Longet S, Austin JA et al (2021) Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 184:5699-5714.e11 Tauzin A, Gong SY, Beaudoin-Bussières G et al (2022) Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe 30:97-109.e5 Parry H, Bruton R, Stephens C et al (2022) Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 7:14 Sallusto F, Lanzavecchia A, Araki K et al (2010) From vaccines to memory and back. Immunity 33:451–463 Thomas SJ, Moreira ED Jr, Kitchin N et al (2021) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 385:1761–1773 Ali H, Alahmad B, Al-Shammari AA et al (2021) Previous COVID-19 infection and antibody levels after vaccination. Front Public Health 9:778243 Vicenti I, Basso M, Gatti F et al (2021) Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose. Int J Infect Dis 112:40–44 Steensels D, Pierlet N, Penders J et al (2021) Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 326:1533–1535References
Acknowledgements
List of GeroCovid Vax Study Group researcher is available at ‘Abbatecola A, Antonelli Incalzi R, Malara A, Palmieri A, Di Lonardo A, Borselli G, Russo M, Noale M, Fumagalli S, Gareri P, Mossello E, Trevisan C, Volpato S, Monzani F, Coin A, Bellelli G, Okoye C, del Signore S, Zia G, & GeroCovid Vax Group. Disentangling the impact of COVID-19 infection on clinical outcomes and preventive strategies in older persons: an Italian perspective. Journal of Gerontology and Geriatrics. 2021 1-9. https://doi.org/10.36150/2499-6564-N440.’ (Ref. 12). We gratefully acknowledge the technical assistance of Daniela Diamanti and Fabiola Diamanti.
Funding
The GeroCovid Vax Study was funded by a grant from the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA – resolution n 14 – feb 4, 2021).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Human and animal rights
The study was conducted in accordance with Helsinki declaration as revised in 2013.
Informed consent
Written informed consent was obtained from the participants of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fedele, G., Palmieri, A., Damiano, C. et al. Humoral immunity induced by mRNA COVID-19 vaccines in Nursing Home Residents previously infected with SARS-CoV-2. Aging Clin Exp Res 34, 2577–2584 (2022). https://doi.org/10.1007/s40520-022-02239-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02239-0