Abstract
Introduction
The inclusion of herbal medicinal products and herbal supplements in pharmacovigilance systems is important because a systematic approach of collecting and analyzing adverse drug reactions related to these products will help practitioners, patients, and regulators to gain more knowledge and prevent harm.
Objective
We aimed to categorize the adverse drug reaction reports on herbal medicinal products and herbal supplements submitted to the Pharmacovigilance Centre Lareb between 1991 and February 2021 on the basis of their regulatory status, herbs included, and adverse drug reactions involved.
Methods
We categorized products on the basis of their registration status and herbal ingredients. The products were then categorized according to the Herbal Anatomical Therapeutic Chemical Classification System. We used descriptive statistics in Microsoft Excel 2019. Pivot tables were used for the analysis and presentation of the data.
Results
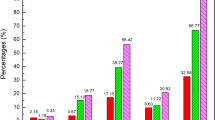
Until February 2021, a total of 789 reports of herbal medicinal products and herbal supplements were received by Lareb. In these reports, a total of 823 herbal products were labeled as suspect. These products caused a total of 1727 adverse drug reactions. Of the 823 products, 229 were registered as a medicine, and 594 were on the market as a herbal supplement. Of the 823 herbal products, 522 reports concerned single-herb products, 256 reports concerned combination products, 27 reports concerned vitamin products containing herbal ingredients, and 18 reports concerned product issues. Approximately 15% of reports concerned serious adverse drug reactions, and adulterated products harbored a high risk of causing serious adverse drug reactions.
Conclusions
Analysis of the herbal medicinal products and herbal supplements in the Dutch pharmacovigilance database revealed a variety of suspected herbal ingredients. The reports provide insight into the variety of herbal products used in the Netherlands and the adverse reactions associated with their use. Pharmacovigilance of herbal products is essential to ensure their safe use.



Similar content being viewed by others
References
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. https://doi.org/10.3389/fphar.2013.00177.
European Medicines Agency (EMA). Herbal medicinal products; 2020. https://www.ema.europa.eu/en/human-regulatory/herbal-medicinal-products. Accessed 11 Nov 2021.
Borg JJ, Laslop A, Pani L, Maciulaitis R, Melchiorri D. Reflections on decisions made on the well-established use of medicinal products by EU regulators and the ECJ. Sci Pharm. 2014;82(3):541–54. https://doi.org/10.3797/scipharm.1402-14.
van Galen E. Traditional herbal medicines worldwide, from reappraisal to assessment in Europe. J Ethnopharmacol. 2014;158 Pt B:498–502. https://doi.org/10.1016/j.jep.2014.07.013.
Kroes BH. The legal framework governing the quality of (traditional) herbal medicinal products in the European Union. J Ethnopharmacol. 2014;158 Pt B:449–53. https://doi.org/10.1016/j.jep.2014.07.044.
de Boer A, Geboers L, van de Koppel S, van Hunsel F. Nutrivigilance: reporting adverse events of non-registered products in the Netherlands. Curr Dev Nutr. 2021;5(Suppl 2):1265. https://doi.org/10.1093/cdn/nzab056_003.
Calapai G. European legislation on herbal medicines: a look into the future. Drug Saf. 2008;31(5):428–31. https://doi.org/10.2165/00002018-200831050-00009.
Rijksoverheid. Warenwetbesluit algemene productveiligheid; 1993. https://wetten.overheid.nl/BWBR0006158/2020-06-09. Accessed 11 May 2022.
Netherlands Enterprise Agency. Commodities Act; 2022. Accessed 25 Mar 2022.
Snyder FJ, Dundas ML, Kirkpatrick C, Neill KS. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81–95. https://doi.org/10.1080/01639360802634043.
Tengku Mohamad TAS, Islahudin F, Jasamai M, Jamal JA. Preference, perception and predictors of herbal medicine use among Malay women in Malaysia. Patient Prefer Adherence. 2019;13:1829–37. https://doi.org/10.2147/ppa.S227780.
Barnes J. Pharmacovigilance of herbal medicines: a UK perspective. Drug Saf. 2003;26(12):829–51.
Menniti-Ippolito F, Mazzanti G, Santuccio C, Moro PA, Calapai G, Firenzuoli F, et al. Surveillance of suspected adverse reactions to natural health products in Italy. Pharmacoepidemiol Drug Saf. 2008;17(6):626–35. https://doi.org/10.1002/pds.1566.
Dutch Medicines Evaluation Board. SmPC Hypericum perforatum Laif®; 2022. https://www.geneesmiddeleninformatiebank.nl/smpc/h103963_smpc.pdf. Accessed 25 Mar 2022.
ICH guideline E2B (R3) on electronic transmission of individual case safety reports (ICSRs): data elements and message specification: implementation guide European Medicines Agency; 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-4.pdf. Accessed 11 May 2022.
Z Index. About Z-index; 2018. https://www.z-indexnl/english. Accessed 11 May 2022.
Hunsel F, Skalli S. Chapter 9. Coding reports involving herbal medicines in a pharmacovigilance database. In: Jo Barnes, editor. Pharmacovigilance of herbal medicines. Springer; 2022 (in press).
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Welcome to MedDRA; 2021. https://www.meddra.org/. Accessed 11 May 2022.
CIOMS Working Group VIII. Practical aspects of signal detection in pharmacovigilance: report of CIOMS Working Group VIII. Report No.: 9290360828, Geneva; 2010.
Farah MH, Edwards R, Lindquist M, Leon C, Shaw D. International monitoring of adverse health effects associated with herbal medicines. Pharmacoepidemiol Drug Saf. 2000;9(2):105–12. https://doi.org/10.1002/(SICI)1099-1557(200003/04)9:2%3c105::AID-PDS486%3e3.0.CO;2-2.
The Herbal Anatomical Therapeutic Chemical Classification System. Uppsala Monitoring Centre. https://www.who-umc.org/whodrug/whodrug-portfolio/whodrug-global/herbal-atc/. Accessed 1 May 2021.
European Medicines Agency (EMA). European Union monographs and list entries; 2022. https://www.ema.europa.eu/en/human-regulatory/herbal-products/european-union-monographs-list-entries#:~:text=A%20European%20Union%20(EU)%20herbal,preparations%20intended%20for%20medicinal%20use. Accessed 25 Mar 2022.
Jeurissen SMF, Buurma-Rethans EJM, Beukers MH, Jansen-van der Vliet M, van Rossum CTM, Sprong RC. Consumption of plant food supplements in the Netherlands. Food Funct. 2018;9(1):179–90. https://doi.org/10.1039/c6fo01174h.
van Hunsel F, Venhuis BJ, Keizers PH, Kant A. A “natural” weight loss product containing sibutramine. Drug Test Anal. 2016;8(3–4):311–4. https://doi.org/10.1002/dta.1925.
van Hunsel F, van Grootheest K. Adverse drug reactions of a slimming product contaminated with sibutramine. Ned Tijdschr Geneeskd. 2011;155(42):A3695.
Kim M, Woo Y, Han C-H. Current status of the spontaneous reporting and classification/coding system for herbal and traditional medicine in pharmacovigilance. Integr Med Res. 2021;10(1): 100467. https://doi.org/10.1016/j.imr.2020.100467.
de Vries ST, Denig P, Ekhart C, Burgers JS, Kleefstra N, Mol PGM, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85(7):1507–15. https://doi.org/10.1111/bcp.13923.
Zhang Y, Leach MJ, Hall H, Sundberg T, Ward L, Sibbritt D, et al. Differences between male and female consumers of complementary and alternative medicine in a national US population: a secondary analysis of 2012 NIHS data. Evid Based Complement Alternat Med. 2015;2015: 413173. https://doi.org/10.1155/2015/413173.
Rhee TG, Harris IM. Gender differences in the use of complementary and alternative medicine and their association with moderate mental distress in U.S. adults with migraines/severe headaches. Headache. 2017;57(1):97–108. https://doi.org/10.1111/head.12986.
Stjernberg L, Berglund J, Halling A. Age and gender effect on the use of herbal medicine products and food supplements among the elderly. Scand J Prim Health Care. 2006;24(1):50–5. https://doi.org/10.1080/02813130500475522.
van Hunsel F, Harmark L, Rolfes L. Fifteen years of patient reporting: what have we learned and where are we heading to? Expert Opin Drug Saf. 2019;18(6):477–84. https://doi.org/10.1080/14740338.2019.1613373.
Walji R, Boon H, Barnes J, Austin Z, Welsh S, Baker GR. Consumers of natural health products: natural-born pharmacovigilantes? BMC Complement Altern Med. 2010;10:8. https://doi.org/10.1186/1472-6882-10-8.
Vlieger AM, van de Putte EM, Hoeksma H. The use of complementary and alternative medicine in children at a general paediatric clinic and parental reasons for use. Ned Tijdschr Geneeskd. 2006;150(11):625–30.
Dauncey EA, Irving JTW, Allkin R. A review of issues of nomenclature and taxonomy of Hypericum perforatum L. and Kew’s medicinal plant names services. J Pharm Pharmacol. 2019;71(1):4–14. https://doi.org/10.1111/jphp.12831.
Chen G, Sun W. The role of botanical gardens in scientific research, conservation, and citizen science. Plant Divers. 2018;40(4):181–8. https://doi.org/10.1016/j.pld.2018.07.006.
Venhuis BJ, van Hunsel F, van de Koppel S, Keizers PH, Jeurissen SM, De Kaste D. Pharmacologically effective red yeast rice preparations marketed as dietary supplements illustrated by a case report. Drug Test Anal. 2016;8(3–4):315–8. https://doi.org/10.1002/dta.1929.
van Hunsel FP, van Grootheest AC. Adverse reactions to herbal remedies: analysis of reported adverse reactions in the Netherlands. Ned Tijdschr Geneeskd. 2013;157(47):A6615.
Vrolijk MF, van de Koppel S, van Hunsel F. Red yeast rice (Monascus purpureus) supplements: case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br J Clin Pharmacol. 2021;87(4):2146–51. https://doi.org/10.1111/bcp.14599.
van Hunsel F, van de Koppel S, van Puijenbroek E. Post-menopausal vaginal hemorrhage related to the use of a hop-containing phytotherapeutic product. Drug Saf Case Rep. 2015;2(1):14. https://doi.org/10.1007/s40800-015-0016-2.
van Hunsel FP, Kampschöer P. Postmenopausal bleeding and dietary supplements: a possible causal relationship with hop- and soy-containing preparations. Ned Tijdschr Geneeskd. 2012;156(41):A5095.
de Boer A, van Hunsel F, Bast A. Adverse food-drug interactions. Regul Toxicol Pharmacol. 2015;73(3):859–65. https://doi.org/10.1016/j.yrtph.2015.10.009.
van de Meerendonk HW, van Hunsel FP, van der Wiel HE. Autoimmune hepatitis induced by Actaea racemosa: side affects of an herb extract. NedTijdschr Geneeskd. 2009;153(6):246–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this study.
Conflict of interest
The authors declare no conflicts of interest that are directly relevant to the content of this study. Florence P.A.M. van Hunsel is a member of the ISoP Special Interest Group on Herbal and Traditional Medicines. This study was presented as a poster at the Annual ISoP Meeting 2021.
Ethics approval
Ethics approval was not needed for this study.
Consent to participate
No approval or consent was needed for this study.
Consent for publication
No approval or consent was needed for this study.
Availability of data and material
The datasets for this manuscript are not publicly available because of the Lareb data-protection policy. Requests to access the datasets should be directed to the first author and will be granted on reasonable request.
Code availability
The SQL statements for the data used in this article are not publicly available because of the Lareb data-protection policy. Requests to access the datasets should be directed to the first author and will be granted on reasonable request.
Author contributions
The original study protocol was designed by all authors. The dataset was established by DK with the help of SK. Data analysis was performed by DK with input from the other authors. The design of the manuscript was determined by all authors. All authors contributed to the final data analysis and to manuscript drafting and revision. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Hunsel, F.P.A.M., van der Kooi, D., van de Koppel, S. et al. Analysis of Reports on Adverse Drug Reactions Related to Herbal Medicinal Products and Herbal Supplements in the Netherlands Received by the National Pharmacovigilance Centre Lareb. Drug Saf 45, 651–661 (2022). https://doi.org/10.1007/s40264-022-01180-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01180-5