Abstract
Multiple sclerosis (MS) is a devastating chronic autoimmune demyelinating disease of the central nervous system (CNS), thought to affect more than 2.5 million people worldwide. Regulation of the sleep-wake cycle might influence disease activity and the frequency of relapses in patients. As melatonin (or sleep hormone) involves the regulation of circadian rhythms, much attention has been paid to the management of MS symptoms with melatonin. This review describes the pharmacological mechanisms underlying the neuroprotective effects of melatonin and recent clinical evidence from MS patients. Apparent risks and benefits of melatonin therapies are also discussed. Various in vivo and clinical data presented in this up-to-date review suggest that melatonin may possibly possess a protective role against the behavioral deficits and neuropathological characteristics of MS. Multiple mechanisms of the neuroprotective effects of melatonin such as mitochondrial protection and antioxidant, anti-inflammatory, and anti-apoptotic properties, as well as its anti-demyelinating function are also discussed. A large body of evidence shows that melatonin potently regulates the immune system, demyelination, free radical generation, and inflammatory responses in neural tissue, which are mediated by multiple signal transduction cascades. In the present article, we focus on different pathways that are targeted by melatonin to prevent the development and progression of MS.


Similar content being viewed by others
References
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545.
Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis—a quiet revolution. Nat Rev Neurol. 2015;11(3):134.
Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72(3–4):132–41.
Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55(4):458–68.
Rahmanzadeh R, Moghadasi AN, Navardi S, Minagar A, Sahraian MA. Multiple sclerosis: clinical features, pathophysiology, diagnosis, and management. In: Minagar A, editor. Neuroinflammation. New York: Elsevier; 2018. p. 1–20.
Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, et al. Role of the blood–brain barrier in multiple sclerosis. Arch Med Res. 2014;45(8):687–97.
Kamphuis WW, Derada Troletti C, Reijerkerk A, Romero IA, de Vries HE. The blood–brain barrier in multiple sclerosis: microRNAs as key regulators. CNS Neurol Disord Drug Targets (Curr Drug Targets CNS Neurol Disord). 2015;14(2):157–67.
Hoffmann K. Photoperiod, pineal, melatonin and reproduction in hamsters. Prog Brain Res. 1979;52:397–415.
Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1(2):109–31.
Goldman BD, Darrow JM. The pineal gland and mammalian photoperiodism. Neuroendocrinology. 1983;37(5):386–96.
Bittman EL, Karsch FJ. Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biol Reprod. 1984;30(3):585–93.
Tamarkin L, Baird CJ, Almeida O. Melatonin: a coordinating signal for mammalian reproduction? Science. 1985;227(4688):714–20.
Pévet P. The role of the pineal gland in the photoperiodic control of reproduction in different hamster species. Reprod Nutr Dev. 1988;28(2B):443–58.
Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16(4):283–301.
Letechipı́a-Vallejo G, González-Burgos I, Cervantes M. Neuroprotective effect of melatonin on brain damage induced by acute global cerebral ischemia in cats. Arch Med Res. 2001;32(3):186–92.
Kennaway DJ, Lushington K, Dawson D, Lack L, van den Heuvel C, Rogers N. Urinary 6-sulfatoxymelatonin excretion and aging: new results and a critical review of the literature. J Pineal Res. 1999;27(4):210–20.
Reiter RJ. The pineal gland and melatonin in relation to aging: a summary of the theories and of the data. Exp Gerontol. 1995;30(3–4):199–212.
Reiter RJ, Tan D-X, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7(6):444–58.
Vaněček J, Pavlík A, Illnerová H. Hypothalamic melatonin receptor sites revealed by autoradiography. Brain Res. 1987;435(1–2):359–62.
Pevet P, Bothorel B, Slotten H, Saboureau M. The chronobiotic properties of melatonin. Cell Tissue Res. 2002;309(1):183–91.
Quay W. Circadian and estrous rhythms in pineal melatonin and 5-hydroxy indole-3-acetic acid. Proc Soc Exp Biol Med. 1964;115(3):710–3.
Cardinali DP, Larin F, Wurtman RJ. Control of the rat pineal gland by light spectra. Proc Natl Acad Sci. 1972;69(8):2003–5.
Brammer GL, Yumiler A, Wetterberg L. N-Acetyltransferase activity of the rat Harderian gland. Biochim Biophys Acta. 1978;526(1):93–9.
Vivien-Roels B, Pevet P, Dubois M, Arendt J, Brown G. Immunohistochemical evidence for the presence of melatonin in the pineal gland, the retina and the Harderian gland. Cell Tissue Res. 1981;217(1):105–15.
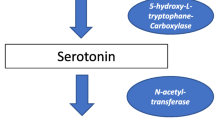
Roseboom P, Coon S, Baler R, McCune S, Weller J, Klein D. Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137(7):3033–44.
Ribelayga C, Gauer F, Pévet P, Simonneaux V. Distribution of hydroxyindole-O-methyltransferase mRNA in the rat brain: an in situ hybridisation study. Cell Tissue Res. 1998;291(3):415–21.
Djeridane Y, Vivien-Roels B, Simonneaux V, Miguez JM, Pévet P. Evidence for melatonin synthesis in rodent Harderian gland: a dynamic in vitro study. J Pineal Res. 1998;25(1):54–64.
Djeridane Y, Pitrosky B, Vivien-Roels B, Simonneaux V, Kirsch R, Pévet P. Long-term daily melatonin infusion induces a large increase in N-acetyltransferase activity, hydroxyindole-O-methyltransferase activity, and melatonin content in the Harderian gland and eye of pinealectomized male Siberian hamsters (Phodopus sungorus). J Pineal Res. 2000;29(2):65–73.
Quay W, Ma Y. Demonstration of gastrointestinal hydroxyindole-O-methyltransferase. IRCS Med Sci. 1976;4:563.
Gauer F, Craft CM. Circadian regulation of hydroxyindole-O-methyltransferase mRNA levels in rat pineal and retina. Brain Res. 1996;737(1–2):99–109.
Pévet P, Balemans M, Legerstee W, Vivien-Roels B. Circadian rhythmicity of the activity of hydroxyindole-O-methyl transferase (HIOMT) in the formation of melatonin and 5-methoxytryptophol in the pineal, retina, and harderian gland of the golden hamster. J Neural Transm. 1980;49(4):229–45.
Dubocovich ML, Takahashi JS. Use of 2-[125I] iodomelatonin to characterize melatonin binding sites in chicken retina. Proc Natl Acad Sci. 1987;84(11):3916–20.
Lopez-Gonzalez M, Calvo J, Rubio A, Goberna R, Guerrero J. Characterization of melatonin binding sites in the Harderian gland and median eminence of the rat. Life Sci. 1991;48(12):1165–71.
Cardinali D, Nagle C, Freire F, Rosner J. Effects of melatonin on neurotransmitter uptake and release by synaptosome-rich homogenates of the rat hypothalamus. Neuroendocrinology. 1975;18(1):72–85.
Carneiro RC, Toffoleto O, Cipolla-Neto J, Marcus RP. Modulation of sympathetic neurotransmission by melatonin. Eur J Pharmacol. 1994;257(1–2):73–7.
Markus RP, Zago WM, Carneiro R. Melatonin modulation of presynaptic nicotinic acetylcholine receptors in the rat vas deferens. J Pharmacol Exp Ther. 1996;279(1):18–22.
Bucher B, Gauer F, Pévet P, Masson-Pévet M. Vasoconstrictor effects of various melatonin analogs on the rat tail artery in the presence of phenylephrine. J Cardiovasc Pharmacol. 1999;33(2):316–22.
Wan Q, Man H-Y, Liu F, Braunton J, Niznik HB, Pang SF, et al. Differential modulation of GABA A receptor function by Mel 1a and Mel 1b receptors. Nat Neurosci. 1999;2(5):401.
Golombek DA, Pévet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev. 1996;20(3):403–12.
Pierpaoli W, Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci. 1994;91(2):787–91.
Menendez-Pelaez A, Reiter RJ. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res. 1993;15(2):59–69.
Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a) anthracene-induced mammary tumors in the rat. Cancer Res. 1981;41(11 Pt 1):4432–6.
Blask D, Hill S. Effects of melatonin on cancer: studies on MCF-7 human breast cancer cells in culture. J Neural Transm Suppl. 1986;21:433–49.
Hill SM, Blask DE. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988;48(21):6121–6.
Teplitzky S, Kiefer T, Cheng Q, Dwivedi P, Moroz K, Myers L, et al. Chemoprevention of NMU-induced rat mammary carcinoma with the combination of melatonin and 9-cis-retinoic acid. Cancer Lett. 2001;168(2):155–63.
Kiefer T, Ram P, Yuan L, Hill S. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res Treat. 2002;71(1):37–45.
Scott AE, Cosma GN, Frank AA, Wells RL, Gardner HS Jr. Disruption of mitochondrial respiration by melatonin in MCF-7 cells. Toxicol Appl Pharmacol. 2001;171(3):149–56.
Pozo D, Reiter RJ, Calvo JR, Guerrero JM. Physiological concentrations of melatonin inhibit nitric oxide synthase in rat cerebellum. Life Sci. 1994;55(24):PL455–60.
Benot S, Gobema R, Reiter RJ, Garcia-Mauriño S, Osuna C, Guerrero JM. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J Pineal Res. 1999;27(1):59–64.
Armstrong S, Redman J. Melatonin: a chronobiotic with anti-aging properties? Med Hypotheses. 1991;34(4):300–9.
Reiter R, Tan D, Mayo J, Sainz R, Leon J, Bandyopadhyay D. Neurally-mediated and neurally-independent beneficial. J Physiol Pharmacol. 2003;54(4):113–25.
Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9.
Stone TW, Forrest CM, Stoy N, Darlington LG. Involvement of kynurenines in Huntington’s disease and stroke-induced brain damage. Neural Transm. 2012;119(2):261–74.
Kwidzinski E, Bunse J Jr, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19(10):1347–9.
Mancuso R, Hernis A, Agostini S, Rovaris M, Caputo D, Fuchs D, et al. Indoleamine 2,3 dioxygenase (IDO) expression and activity in relapsing-remitting multiple sclerosis. PLoS ONE. 2015;10(6):e0130715.
Sakurai K, Zou J-P, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129(1–2):186–96.
Sundaram G, Brew BJ, Jones SP, Adams S, Lim CK, Guillemin GJ. Quinolinic acid toxicity on oligodendroglial cells: relevance for multiple sclerosis and therapeutic strategies. J Neuroinflamm. 2014;11(1):204.
Anderson G, Rodriguez M. Multiple sclerosis, seizures, and antiepileptics: role of IL-18, IDO, and melatonin. Eur J Neurol. 2011;18(5):680–5.
Zhou H, Wang J, Jiang J, Stavrovskaya IG, Li M, Li W, et al. N-Acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J Neurosci. 2014;34(8):2967–78.
Klein DC, Coon SL, Roseboom PH, Weller J, Bernard M, Gastel JA, et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Hormone Res. 1997;52:307–58.
Khalil EM, De Angelis J, Ishii M, Cole PA. Mechanism-based inhibition of the melatonin rhythm enzyme: pharmacologic exploitation of active site functional plasticity. Proc Natl Acad Sci. 1999;96(22):12418–23.
Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26(8):412–9.
Ferry G, Mozo J, Ubeaud C, Berger S, Bertrand M, Try A, et al. Characterization and regulation of a CHO cell line stably expressing human serotonin N-acetyltransferase (EC 2.3. 1.87). Cell Mol Life Sci CMLS. 2002;59(8):1395–405.
Ganguly S, Coon SL, Klein DC. Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell Tissue Res. 2002;309(1):127–37.
Garbarino-Pico E, Carpentieri AR, Contin MA, Sarmiento MIK, Brocco M, Panzetta P, et al. Retinal ganglion cells are autonomous circadian oscillators synthesizing N-acetylserotonin during the day. J Biol Chem. 2004;279(49):51172–81.
Hirata F, Hayaishi O, Tokuyama T, Senoh S. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974;249(4):1311–3.
Takikawa O, Yoshida R, Hayaishi O. Monooxygenase activities of dioxygenases. Benzphetamine demethylation and aniline hydroxylation reactions catalyzed by indoleamine 2,3-dioxygenase. J Biol Chem. 1983;258(11):6808–15.
Allegra M, Furtmüller PG, Regelsberger G, Turco-Liveri ML, Tesoriere L, Perretti M, et al. Mechanism of reaction of melatonin with human myeloperoxidase. Biochem Biophys Res Commun. 2001;282(2):380–6.
de Oliveira Silva S, **menes VF, Catalani LH, Campa A. Myeloperoxidase-catalyzed oxidation of melatonin by activated neutrophils. Biochem Biophys Res Commun. 2000;279(2):657–62.
Ferry G, Ubeaud C, Lambert P-H, Bertin S, Francis C, Chomarat P, et al. Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem J. 2005;388(1):205–15.
Li Y, Hu N, Yang D, Oxenkrug G, Yang Q. Regulating the balance between the kynurenine and serotonin pathways of tryptophan metabolism. FEBS J. 2017;284(6):948–66.
Yang Y, Duan W, ** Z, Yi W, Yan J, Zhang S, et al. JAK 2/STAT 3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res. 2013;55(3):275–86.
Iguchi H, Kato K-I, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab. 1982;55(1):27–9.
Vakkuri O, Leppäluoto J, Kauppila A. Oral administration and distribution of melatonin in human serum, saliva and urine. Life Sci. 1985;37(5):489–95.
Yeleswaram K, McLaughlin LG, Knipe JO, Schabdach D. Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications. J Pineal Res. 1997;22(1):45–51.
Fourtillan J, Brisson A, Gobin P, Ingrand I, Decourt JP, Girault J. Bioavailability of melatonin in humans after day-time administration of D7 melatonin. Biopharm Drug Dispos. 2000;21(1):15–22.
Flo A, Cambras T, Díez-Noguera A, Calpena A. Melatonin pharmacokinetics after transdermal administration changes according to the time of the day. Eur J Pharm Sci. 2017;96:164–70.
Morgan L, Arendt J, Owens D, Folkard S, Hampton S, Deacon S, et al. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. J Endocrinol. 1998;157(3):443–51.
Sharma S, Singh H, Ahmad N, Mishra P, Tiwari A. The role of melatonin in diabetes: therapeutic implications. Arch Endocr Metab. 2015;59(5):391–9.
Mao S, Chen J, Wei Z, Liu H, Bi D. Intranasal administration of melatonin starch microspheres. Int J Pharm. 2004;272(1–2):37–43.
Bellapart J, Roberts JA, Appadurai V, Wallis SC, Nuñez-Nuñez M, Boots RJ. Pharmacokinetics of a novel dosing regimen of oral melatonin in critically ill patients. Clin Chem Lab Med (CCLM). 2016;54(3):467–72.
Reiter RJ, Guerrero JM, Escames G, Pappolla MA, Acuña‐Castroviejo D. Prophylactic actions of melatonin in oxidative neurotoxicity. Ann N Y Acad Sci. 1997;825(1):70–8.
Leker R, Teichner A, Lavie G, Shohami E, Lamensdorf I, Ovadia H. The nitroxide antioxidant tempol is cerebroprotective against focal cerebral ischemia in spontaneously hypertensive rats. Exp Neurol. 2002;176(2):355–63.
Watson N, Diamandis T, Gonzales-Portillo C, Reyes S, Borlongan CV. Melatonin as an antioxidant for stroke neuroprotection. Cell Transplant. 2016;25(5):883–91.
Lee MY, Kuan YH, Chen HY, Chen TY, Chen ST, Huang CC, et al. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J Pineal Res. 2007;42(3):297–309.
Gomaa AM, Galal HM, Abou-Elgait AT. Neuroprotective effects of melatonin administration against chronic immobilization stress in rats. Int J Physiol Pathophysiol Pharmacol. 2017;9(2):16.
Jacob S, Poeggeler B, Weishaupt JH, Sirén AL, Hardeland R, Bähr M, et al. Melatonin as a candidate compound for neuroprotection in amyotrophic lateral sclerosis (ALS): high tolerability of daily oral melatonin administration in ALS patients. J Pineal Res. 2002;33(3):186–7.
Chahbouni M, Escames G, Venegas C, Sevilla B, García JA, López LC, et al. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res. 2010;48(3):282–9.
Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Melatonin therapy in patients with Alzheimer’s disease. Antioxidants. 2014;3(2):245–77.
Pappolla M, Bozner P, Soto C, Shao H, Robakis NK, Zagorski M, et al. Inhibition of Alzheimer β-fibrillogenesis by melatonin. J Biol Chem. 1998;273(13):7185–8.
Lima ACP, Louzada PR, De Mello FG, Ferreira ST. Neuroprotection against Aβ and glutamate toxicity by melatonin: Are GABA receptors involved? Neurotox Res. 2003;5(5):323–7.
Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003;35(2):125–30.
Giusti P, Lipartiti M, Franceschini D, Schiavo N, Floreani M, Manev H. Neuroprotection by melatonin from kainate-induced excitotoxicity in rats. FASEB J. 1996;10(8):891–6.
Manev H, Uz T, Kharlamov A, Joo J. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. FASEB J. 1996;10(13):1546–51.
Cho S, Joh TH, Baik HH, Dibinis C, Volpe BT. Melatonin administration protects CA1 hippocampal neurons after transient forebrain ischemia in rats. Brain Res. 1997;755(2):335–8.
Kilic E, Öuzdemir YG, Bolay H, Keleştimur H, Dalkara T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J Cereb Blood Flow Metab. 1999;19(5):511–6.
Deng Y-Q, Xu G-G, Duan P, Zhang Q, Wang J-Z. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol Sin. 2005;26(5):519.
Lau WW, Ng JK, Lee MM, Chan AS, Wong YH. Interleukin-6 autocrine signaling mediates melatonin MT1/2 receptor-induced STAT3 Tyr705 phosphorylation. J Pineal Res. 2012;52(4):477–89.
Chuang JI, Mohan N, Meltz ML, Reiter RJ. Effect, of melatonin, on NF-κb dna-binding activity in the rat spleen. Cell Biol Int. 1996;20(10):687–92.
Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, et al. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res. 2004;37(2):129–36.
Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol. 2010;8(3):218–27.
Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. 2009;373(9662):482–91.
McKenna JT, Christie MA, Jeffrey BA, McCoy JG, Lee E, Connolly NP, et al. Chronic ramelteon treatment in a mouse model of Alzheimer’s disease. Arch Ital Biol. 2012;150(1):5.
Mack JM, Schamne MG, Sampaio TB, Pértile RAN, Fernandes PACM, Markus RP, et al. Melatoninergic system in Parkinson’s disease: from neuroprotection to the management of motor and nonmotor symptoms. Oxid Med Cell Longev. 2016;2016:3472032.
Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J Pineal Res. 2011;50(2):97–109.
Wilkinson D, Shepherd E, Wallace EM. Melatonin for women in pregnancy for neuroprotection of the fetus. Cochrane Libr. 2016;3:CD010527.
Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI. Therapeutic application of melatonin in mild cognitive impairment. Am J Neurodegener Dis. 2012;1(3):280.
Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res. 2013;23(3):267–300.
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014. https://doi.org/10.1212/wnl.0000000000000560.
Graetz C, Groppa S, Zipp F, Siller N. Preservation of neuronal function as measured by clinical and MRI endpoints in relapsing-remitting multiple sclerosis: how effective are current treatment strategies? Expert Rev Neurother. 2018;18(3):203–19.
Compston A, Coles A. Multiple sclerosis. Lancet (London, England). 2008;372(9648):1502–17 (Epub 2008/10/31).
Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2 Suppl):S15–20.
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210–8.
Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. 2018;141(7):1900–16.
Bannerman P. Cortical involvement in multiple sclerosis. In: Cechetto D, Weishaupt N, editors. The cerebral cortex in neurodegenerative and neuropsychiatric disorders: New York: Elsevier; 2017. pp. 243–73.
Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–93.
Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89(2):225–40.
Farzaei MH, Shahpiri Z, Bahramsoltani R, Najafi F, Rahimi R. Efficacy and tolerability of phytomedicines in multiple sclerosis patients: a review. CNS Drugs. 2017;31(10):867–89.
Sand IK. The role of diet in multiple sclerosis: mechanistic connections and current evidence. Curr Nutr Rep. 2018;7(3):150–60.
Hart BA. Why does multiple sclerosis only affect human primates? Multip Scler. (Houndmills, Basingstoke, England). 2016;22(4):559–63 (Epub 2015/11/06).
Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164(4):1079–106 (Epub 2011/03/05).
Torkildsen O, Brunborg LA, Myhr KM, Bo L. The cuprizone model for demyelination. Acta Neurol Scand Suppl. 2008;188:72–6 (Epub 2008/06/18).
Vakilzadeh G, Khodagholi F, Ghadiri T, Ghaemi A, Noorbakhsh F, Sharifzadeh M, et al. The effect of melatonin on behavioral, molecular, and histopathological changes in cuprizone model of demyelination. Mol Neurobiol. 2016;53(7):4675–84 (Epub 2015/08/28).
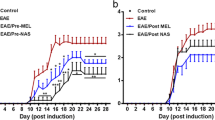
Wen J, Ariyannur PS, Ribeiro R, Tanaka M, Moffett JR, Kirmani BF, et al. Efficacy of N-acetylserotonin and melatonin in the EAE model of multiple sclerosis. J Neuroimmune Pharmacol. 2016;11(4):763–73.
Alvarez-Sanchez N, Cruz-Chamorro I, Lopez-Gonzalez A, Utrilla JC, Fernandez-Santos JM, Martinez-Lopez A, et al. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun. 2015;50:101–14 (Epub 2015/07/03).
Kang JC, Ahn M, Kim YS, Moon C, Lee Y, Wie MB, et al. Melatonin ameliorates autoimmune encephalomyelitis through suppression of intercellular adhesion molecule-1. J Vet Sci. 2001;2(2):85–9 (Epub 2003/11/14).
Long T, Yang Y, Peng L, Li Z. Neuroprotective effects of melatonin on experimental allergic encephalomyelitis mice via anti-oxidative stress activity. J Mol Neurosci MN. 2018;64(2):233–41 (Epub 2018/02/17).
Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251(3):261–8 (Epub 2004/03/12).
van Horssen J, Schreibelt G, Bo L, Montagne L, Drukarch B, van Muiswinkel FL, et al. NAD(P)H:quinone oxidoreductase 1 expression in multiple sclerosis lesions. Free Radic Biol Med. 2006;41(2):311–7 (Epub 2006/07/04).
Arnold P, Mojumder D, Detoledo J, Lucius R, Wilms H. Pathophysiological processes in multiple sclerosis: focus on nuclear factor erythroid-2-related factor 2 and emerging pathways. Clin Pharmacol Adv Appl. 2014;6:35–42 (Epub 2014/03/05).
Ghareghani M, Zibara K, Sadeghi H, Farhadi N. Spasticity treatment ameliorates the efficacy of melatonin therapy in experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis. Cell Mol Neurobiol. 2018;38(5):1145–51 (Epub 2018/03/03).
Kashani IR, Rajabi Z, Akbari M, Hassanzadeh G, Mohseni A, Eramsadati MK, et al. Protective effects of melatonin against mitochondrial injury in a mouse model of multiple sclerosis. Exp Brain Res. 2014;232(9):2835–46 (Epub 2014/05/07).
Campbell GR, Mahad DJ. Mitochondria as crucial players in demyelinated axons: lessons from neuropathology and experimental demyelination. Autoimmune Dis. 2011;2011:262847.
Campbell G, Mahad DJ. Mitochondrial dysfunction and axon degeneration in progressive multiple sclerosis. FEBS Lett. 2018;592(7):1113–21 (Epub 2018/02/18).
Lin W, Lin Y. IFN-γ inhibits central nervous system myelination through both STAT1-dependent and STAT1-independent pathways. J Neurosci Res. 2010;88(12):2569–77.
Chen SJ, Huang SH, Chen JW, Wang KC, Yang YR, Liu PF, et al. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2016;31:169–77 (Epub 2016/01/07).
Ghareghani M, Dokoohaki S, Ghanbari A, Farhadi N, Zibara K, Khodadoust S, et al. Melatonin exacerbates acute experimental autoimmune encephalomyelitis by enhancing the serum levels of lactate: a potential biomarker of multiple sclerosis progression. Clin Exp Pharmacol Physiol. 2017;44(1):52–61 (Epub 2016/10/04).
Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):37–53.
Peschl P, Bradl M, Hoftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529 (Epub 2017/05/24).
Gupta S, Ahsan I, Mahfooz N, Abdelhamid N, Ramanathan M, Weinstock-Guttman B. Osteoporosis and multiple sclerosis: risk factors, pathophysiology, and therapeutic interventions. CNS Drugs. 2014;28(8):731–42 (Epub 2014/05/30).
Ghareghani M, Scavo L, Arnoult D, Zibara K, Farhadi N. Melatonin therapy reduces the risk of osteoporosis and normalizes bone formation in multiple sclerosis. Fundam Clin Pharmacol. 2018;32(2):181–7.
Natarajan R, Einarsdottir E, Riutta A, Hagman S, Raunio M, Mononen N, et al. Melatonin pathway genes are associated with progressive subtypes and disability status in multiple sclerosis among Finnish patients. J Neuroimmunol. 2012;250(1–2):106–10.
Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci. 2012;314(1–2):37–40.
Adamczyk-Sowa M, Sowa P, Adamczyk J, Niedziela N, Misiolek H, Owczarek M, et al. Effect of melatonin supplementation on plasma lipid hydroperoxides, homocysteine concentration and chronic fatigue syndrome in multiple sclerosis patients treated with interferons-beta and mitoxantrone. J Physiol Pharmacol. 2016;67:235–42.
Roostaei T, Sahraian MA, Hajeaghaee S, Gholipour T, Togha M, Siroos B, et al. Impact of melatonin on motor, cognitive and neuroimaging indices in patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2015;14(6):589–95.
Gholipour T, Ghazizadeh T, Babapour S, Mansouri B, Ghafarpour M, Siroos B, et al. Decreased urinary level of melatonin as a marker of disease severity in patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2015;14(1):91–7.
Miller E, Walczak A, Majsterek I, Kedziora J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol. 2013;257(1–2):97–101 (Epub 2013/03/23).
Adamczyk-Sowa M, Galiniak S, Żyracka E, Grzesik M, Naparło K, Sowa P, et al. Oxidative modification of blood serum proteins in multiple sclerosis after interferon beta and melatonin treatment. Oxid Med Cell Longev. 2017;2017:7905148.
Adamczyk-Sowa M, Pierzchala K, Sowa P, Polaniak R, Kukla M, Hartel M. Influence of melatonin supplementation on serum antioxidative properties and impact of the quality of life in multiple sclerosis patients. J Physiol Pharmacol. 2014;65(4):543–50.
Adamczyk-Sowa M, Sowa P, Mucha S, Zostawa J, Mazur B, Owczarek M, et al. Changes in serum ceruloplasmin levels based on immunomodulatory treatments and melatonin supplementation in multiple sclerosis patients. Med Sci Monit Int Med J Exp Clin Res. 2016;22:2484.
Emamgholipour S, Hossein-nezhad A, Sahraian MA, Askarisadr F, Ansari M. Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci. 2016;145:34–41.
Ghorbani A, Salari M, Shaygannejad V, Norouzi R. The role of melatonin in the pathogenesis of multiple sclerosis: a case-control study. Int J Prev Med. 2013;4(Suppl 2):S180.
Mrowicka M, Garncarek P, Miller E, Kedziora J, Smigielski J, Malinowska K, et al. Effect of melatonin on activity of superoxide dismutase (CuZn-SOD) in erythrocytes of patients during short- and long-term hypokinesis. Wiadomosci Lekarskie (Warsaw, Poland: 1960). 2010;63(1):3–9.
Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell. 2015;162(6):1338–52.
Alvarez-Sanchez N, Cruz-Chamorro I, Diaz-Sanchez M, Sarmiento-Soto H, Medrano-Campillo P, Martinez-Lopez A, et al. Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J Pineal Res. 2017;63(4):e12442 (Epub 2017/08/10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source funding was used to prepare this review.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yeganeh Salehpour, M., Mollica, A., Momtaz, S. et al. Melatonin and Multiple Sclerosis: From Plausible Neuropharmacological Mechanisms of Action to Experimental and Clinical Evidence. Clin Drug Investig 39, 607–624 (2019). https://doi.org/10.1007/s40261-019-00793-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00793-6