Abstract
Purpose of Review
We review the latest developments in restoration of lacrimal gland function in dry eye disease and promising methods to generate functional lacrimal gland units.
Recent Findings
Mesenchymal stem cell delivery demonstrated improved tear secretion in dry eye mouse models and early human studies, likely through immune modulation and lacrimal gland repair mechanisms. Advances in regenerative strategies to create functional lacrimal gland units included new porcine scaffolds, the organ germ method, novel methods to generate lacrimal organoids, and 3-dimensional bioprinting. FGF signaling holds an important role in the development and growth of lacrimal gland epithelium.
Summary
Advances in the various approaches to restoring function and engineering lacrimal units show promise for future clinical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dry eye disease (DED) causes a significant toll on patients, with a prevalence of approximately 22 million American adults, and is associated with a significant healthcare cost of approximately $3.84 billion in the US in 2008 [1]. DED is associated with worse visual function, activity limitations, and lower socioeconomic functioning [2]. The severity of DED symptoms is directedly related to the impact on vision-related quality of life [2].
DED may result from aqueous tear deficiency, evaporative disease, or a combination of these processes [3]. Treatment requires frequent, oftentimes burdensome, administration of artificial tears or other topical agents. Chronic, severe cases of DED that are inadequately treated may lead to decreased vision, reduced quality of life, persistent epithelial defects, epithelial dysfunction, and poor wound healing, with potential consequences including corneal ulcers or permanent vision loss. Current treatments are based on symptom management and require chronic frequent administration of topical agents to reduce inflammation or palliate [1, 4]. Procedures such as intense pulsed light therapy and LipiFlow (TearScience, Morrisville, NC) have varying rates of success and duration of disease control and are not standards of care [1]. Movement towards restoration of ocular surface homeostasis through regeneration of lacrimal gland secretory function remains elusive, but exciting progress has been made.
Research into lacrimal gland regeneration has remained an active field over the last two decades and may offer a more permanent treatment modality for dry eye disease, especially in the setting of aqueous deficiency [5]. This review focuses on recent advances in the past five years that show promise for future translational applications, including progress in the areas of stem cell replacements, tissue engineering, generation of functional, lacrimal-gland-like organoids, and growth factor modulation to stimulate endogenous lacrimal gland function.
Mesenchymal Stem Cells in Lacrimal Gland Regeneration
Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent progenitor cells capable of self-renewal [6, 7]. Adult MSCs isolated from bone marrow are considered the gold standard; however, adipose tissue, peripheral blood, and human exfoliated deciduous teeth (SHED) have been shown to be adequate sources of MSCs (Fig. 1) [8, 9]. MSCs have also been cultured from human lacrimal gland tissue obtained from surgery [10]. MSCs derived from lacrimal gland tissue express characteristic MSC cell surface markers and have similar spindle-shaped morphology and colony-formation characteristics to MSCs derived from bone marrow [10]. MSCs can differentiate into specialized cell types such as bone and muscle and are also highly active in tissue repair and maintenance with known immunomodulatory and anti-inflammatory roles demonstrated in mouse models and human inflammatory or ischemic disease [7, 11,12,13]. In lacrimal gland regeneration, prior work has shown that MSCs can be directed towards lacrimal gland epithelial regeneration [5]. In the past several years, research has been aimed at the delivery of MSCs to the site of lacrimal gland injury, the effects of this on cellular repair and regeneration, and potential mechanisms.
Mesenchymal stem cells in lacrimal gland regeneration. Mesenchymal stem cells may be harvested from various sites including bone marrow, blood, adipose, teeth, and lacrimal gland. In mouse models of dry eye disease, delivery of MSCs lead to improved survival and decreased markers of inflammation in lacrimal tissue. Genes associated with lacrimal gland regeneration, proliferation, and tear secretion showed increase expression. MSC: mesenchymal stem cell
Delivery of Mesenchymal Stem Cells in Disease Models
Several disease models have been used in the study of lacrimal gland regeneration. These include Murphy Roths Large (MRL) lymphoproliferative mice used to study dry eye in Sjögren’s syndrome, and non-obese diabetic (NOD) mice, which are established models of insulin-dependent diabetes mellitus that also are used to model dry eye from Sjögren’s syndrome. Lacrimal duct-ligated mice and lacrimal gland-excised mice have also been used to study aqueous-deficient dry eye disease [14].
In experimental models, human or mouse-derived MSCs are delivered by subconjunctival or direct lacrimal gland injection. Tail vein and intraperitoneal injections likely localize to the site of lacrimal gland injury after entering the circulation [15].
Effect of Mesenchymal Stem Cells on Tear Secretion
While studies using MSC techniques have been ongoing since 2010, interest in the past 5 years has continued to grow with increasing data suggesting the potential of MSCs in improving tear secretion, including increased tear production, repair and proliferation of injured lacrimal tissue, and alterations in the inflammatory profile. Mouse bone marrow-derived mesenchymal stem cells (BD-MSCs) delivered to NOD mice showed increased tear production from baseline compared to phosphate-buffered saline (PBS) delivery [6]. Human-derived MSCs delivered by subconjunctival and direct lacrimal gland injection in NOD mice have also been shown to improve tear secretion 14 days after injection compared to PBS-injected mice [16]. This was associated with twice as much lacrimal gland goblet cell content compared to PBS-injected mice shown on histology [16]. In a separate study utilizing MSC lysate, the thicknesses of the corneal epithelium in these mice were significantly higher than in control mice, suggesting preserved corneal integrity and overall health likely secondary to preserved tear flow over an extended period of time [7].
Based on encouraging results from animal studies, reports of general tolerability with human MSC delivery for heart disease, and clinically functional improvement in osteoarthritis, the use of MSCs in humans for aqueous-deficient dry eye has recently been explored [17, 18]. In an open-label clinical trial, seven participants with aqueous-deficient DED due to either primary or secondary Sjögren’s syndrome received one transconjunctival injection of allogenic adipose-derived mesenchymal stem cells into the palpebral lobe of the lacrimal gland of one eye [19]. At 16 weeks, there was a statistically significant decrease in the mean Ocular Surface Disease Index score from 58.9 at baseline to 34.1. In addition, the mean tear osmolarity in the treated eye decreased from 312.9 to 291.6 mosm/L, the mean tear film break up time (TBUT) increased from 3.7 to 7.1, and the mean Schirmer’s I test increased from 4.6 to 8.1 mm/5 min in the MSC injected eye [19]. Of these parameters, post-injection measurements found tear osmolarity to be significantly lower and TBUT scores higher in the injected MSC-injected eye compared to the non-injected eye [19]. No adverse events related to the study treatment were reported. Although studies assessing MSC injections in humans are currently limited, the results are promising and pave the way for future studies in human participants.
MSCs Role in Lacrimal Gland Tissue Repair
Functional studies showing improvement in tear secretion after MSC treatment have been supported by histologic and molecular studies of the lacrimal gland structure and composition. Immunofluorescence staining of mice lacrimal glands after MSC and MSC extract treatment showed significant elevations in markers for lacrimal acini (AQP5 and AQP4), myoepithelial cells (alpha Smooth Muscle Actin), ductal/progenitor cells (cytokeratin 5), and stem/progenitor cells (c-Kit) compared to controls [7]. This was associated with higher proliferation rates, measured by Ki-67 expression, and elevations in serum epidermal growth factor (EGF), at a time point that coincided with pronounced tear secretion rates relative to controls [7]. Additional genes associated with regeneration, proliferation, and glandular secretion – EGF, fibroblast growth factor 2 (FGF2), lysozyme 1 (LYZ1), and AQP5 – were also upregulated in MSC-treated lacrimal glands [7]. Bone morphogenic protein 7 (BMP7), a growth factor associated with extracellular matrix remodeling in injury, was also upregulated [7, 20].
Mice studies of transplantation of sex-mismatched, e-GFP expressing MSCs showed that MSCs were detected in lacrimal glands but dwindled over 21 days [21]. Thus, improvement in vital lacrimal gland structures after MSC transplantation was likely due to the secretion of trophic factors, as opposed to direct engraftment [21]. In an in vitro study evaluating secretomes from isolated murine lacrimal gland MSCs, several factors were identified as having potential therapeutic roles in improving lacrimal gland epithelial cell survival [21]. These included Lipocalin-2, prosaposin, RAS GTPase-activating protein-binding protein 1 (Rac1), and signal transducer and activator of transcription 1 (STAT1) [21]. Additional in vivo studies will help address MSCs’ potential for therapeutic use to induce or enhance lacrimal gland regeneration.
MSC Modulation of Inflammation
In addition to initiating endogenous tissue repair mechanisms, there has been evidence that additional therapeutic roles of MSCs include the ability to suppress inflammation. This was supported by mouse models showing smaller foci of lymphocytic infiltrates by 40.5% compared to control Sjögren’s syndrome NOD mice, reduced macrophage infiltration, and TNF-α expression in the early regenerative phases after MSC treatment [6, 7, 21]. mRNA expression of pro-inflammatory cytokines, TGF-beta, IL-1β, IFN-γ, and IL-17 A, were also downregulated compared to controls (Fig. 1) [7, 16]. In addition, an upregulation of anti-inflammatory cytokine, IL-10 was noted in the serum and lacrimal gland tissue (Fig. 1) [7]. AQP5, a transmembrane water channel involved with glandular secretion that had a 3.5-fold increase in expression levels in MSC-treated mice, has been shown to play a dual role in modulating inflammation [6, 22]. The downregulation of inflammatory cytokine gene expression, upregulation in anti-inflammatory factors, decrease in lacrimal gland lymphocytic influx, and selective suppression of B-cells support a possible secondary mechanism of MSCs in improving peripheral tolerance to prevent immune attack [7].
Generating a three-dimensional Lacrimal Gland through Tissue Engineering
Another approach to treating aqueous-deficiency DED has involved tissue engineering of the lacrimal gland, in which tissue grown in vitro may be transplanted and function synergistically with native tissue. Approaches described included 3-dimensional scaffolds on which lacrimal gland epithelial cells grew, the organ germ method, and various innovations in culturing 3-dimensional gland-like structures (spheroids and organoids) (Fig. 2).
Tissue engineering strategies in generation of 3-dimensional lacrimal gland. A. Scaffolds serve as a support framework for lacrimal gland cells, which enable growth into a complex 3D structure. B. The organ germ method. Lacrimal gland organ germs are harvested from mouse embryos, which are separated into epithelial and mesenchymal stem cells. These cell populations are then co-cultured into a lacrimal germ, which may subsequently be transplanted into gland-excised mice. C. General concept of generating a lacrimal gland-like organoid. Cells from native lacrimal gland tissue or iPSCs may serve as precursor cells towards the growth of spheroids and organoids resembling lacrimal glands. This process aims to generate a functional D lacrimal gland. MSC: mesenchymal stem cell; iPSC: induced pluripotent stem cell; ECM: extracellular matrix
Scaffolds in Lacrimal Gland Tissue Engineering
Scaffolds, cells, and growth-stimulating agents are essential to tissue engineering. Scaffolds provide a structure for cultured cells to attach and develop into desired 3D tissue and are commonly made of a synthetic polymer that promotes cell adhesion, compatible with cell growth, high pore-space, high surface-area to volume ratio, and can withstand in vivo stresses [23, 24] (Fig. 2A). Lin et al. reported a method in which commercially available rabbit lacrimal glands were decellularized and served as a scaffold on which cells from rabbit lacrimal gland explants may be seeded and grown in vitro [25].
Recent innovations in the design of scaffolds in lacrimal gland regeneration included the use of decellularized porcine jejunum (SIS-Muc) as a scaffold for lacrimal gland epithelial cells. Survival and proliferation of lacrimal gland epithelial cell cultures have been demonstrated with SIS-Muc scaffolds. Immunohistochemistry demonstrated similar structural components as native lacrimal gland acini, including epithelial origin and polarization, and functional elements, including secretory vesicles, mucins, lactoferrin, and lysozyme [26]. SIS-Muc was harvested with intact vasculature, which allowed for reseeding with endothelial cells to restore functionality [26]. Recent development has increased the cell count supported by the scaffold, thus potentially facilitating upscaling to sizes relevant for clinical use [26]. These studies support the use of SIS-Muc as a potential scaffold for the growth of lacrimal gland cell populations in the reconstruction of lacrimal gland tissue.
The Organ Germ Method
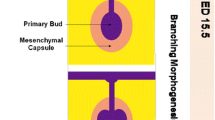
The organ germ method involves utilizing tissue dissected from mouse model embryos to produce 3D organ germs consisting of epithelial and mesenchymal cells that are capable of compartmentalization and can subsequently be transplanted into mouse models [27] (Fig. 2B). In the lacrimal gland organ germ method, lacrimal gland epithelial and mesenchymal tissues were extracted from C57BL/6 strain mice embryos; these cells were co-cultured in vitro to generate lacrimal gland germs with branching morphology [28]. The lacrimal gland germs were successfully transplanted into gland-excised mice with reconnection of the bioengineered lacrimal gland and the native recipient lacrimal excretory duct. Histological analyses of the transplanted bioengineered glands confirmed expression of native lacrimal gland structural and secretary compounds [28]. Engrafted bioengineered lacrimal glands also responded to nervous stimuli and had secretory capacity equivalent to normal lacrimal glands as demonstrated by Western blot analysis showing presence of lactoferrin in tear fluid secretions of both gland-engrafted and normal healthy mice [29]. Additionally, reverse-phase liquid chromatography revealed tear fluid secreted by gland-engrafted mice contained greater amounts of alkyl triglycerides (lipids found in tears) than in normal controls [29]. Corneal thickness measurements were comparable between gland-engrafted and normal mice, while corneal thinning was observed in mice status post gland excision without subsequent engraftment [29]. These findings suggest that gland-engrafted mice produced adequate tears to protect against corneal thinning. The success of engrafted lacrimal gland organ germs in mimicking and restoring lacrimal function in mice provided proof of concept as a potentially viable strategy applicable to dry eye disease treatment.
Development of three-dimensional (3D) Lacrimal Gland Organoids
Advances in stem cell technology have also enabled production of more robust organized 3D aggregates of cells that resemble, and to an extent, perform specific functions of an organ, known as “organoids” or “spheroids”. Organoids are 3D structural units with structure and function that closely resemble an organ [30]. Spheroids are free-floating cellular aggregates of low complexity and resemble the organ to a lesser extent but are generally simpler and less resource-intensive to maintain compared to organoids.
Lacrimal gland spheroids have been generated from both animal and human donors (Fig. 2C). Spheroids have been produced from porcine lacrimal gland epithelial cells and MSCs with human foreskin endothelial cells [31]. MSC and epithelial cells from porcine lacrimal glands and endothelial cells from human foreskin were combined and formed spheroids with relatively increased viability when grown on SIS-Muc scaffolds compared to Matrigel scaffolds, with origin, function, and viability of the cultured cells confirmed by positive expression of pan-cytokeratin, lysozyme, Rab3D, and reduced expression of caspase-3. Lacrimal gland spheroids have also been generated from human cadaveric and adult human lacrimal gland tissue biopsies [32, 33]. These spheroids were shown to have actively dividing cells confirmed with BrdU assays, a similar percentage of cells in the G0/G1 phase compared to native human lacrimal gland tissue, presence of secreted lactoferrin and lysozyme, and expression of AQP5, mucin-5ac (MUC-5ac), and lysozyme, indicating the ability for these spheroids to synthesize and secrete proteins of a normal functional lacrimal gland [32].
Lacrimal gland organoids, which are of higher complexity than spheroids, have also been developed (Fig. 2C). Jeong et al. developed organoids from human lacrimal gland tissue, confirmed by expression of secretory products and gland-specific markers consistent with acinar cells [34]. Administration of pilocarpine increased levels of internal Ca2+ ions, successfully stimulated tear production, and tear proteins were seen on transmission electron microscopy [34]. Successful engraftment of lacrimal organoids derived from transgenic GFP-expressing mice to non-GFP expressing C57BL/6 mice with concanavalin A-induced DED has been shown, indicated by GFP expression detected 14 days post-transplantation [34]. Successful engraftment was further supported by AQP5 gene expression [34]. Several factors have been implicated in aiding successful engraftment - EpCAM, an epithelial lineage cell marker, was found to be instrumental in organoid development, as EpCAM-negative cells were unable to form organoids, and the absence of PAX6 resulted in reduced organoid cell proliferation [34].
While multiple studies have investigated organoid development from animal or human donor tissue, human induced pluripotent stem cells (iPSCs) may also be used as a source for lacrimal gland organoids (Fig. 2C). A multi-zone approach has been described, in which induced iPSCs formed zones of different ectoderm cell types with various morphologies committed to the ocular lineage, leading to the successful growth of lacrimal gland organoids [35, 36]. Successful differentiation and function were confirmed through the detection of mRNA expression of AQP5 as well as the production of key tear proteins such as lysozyme, lactoferrin, and tear lipocalins. Thus, successful lacrimal gland organoid development may arise from a variety of sources, including human and animal donor cells as well as iPSCs.
While xenogenic and synthetic 3D scaffolds show promise, some theoretical disadvantages include its degradation and immunogenic risks, difficulty controlling lacrimal gland size and shape, and maintenance of culture viability [33, 37]. Magnetic 3D bioprinting has been described as a xenogenic-free and highly producible method for generating exocrine gland spheroids and organoids. In this method, biocompatible magnetic nanoparticles were used to tag cells for printing into a desired 3D organization [37]. Although magnetic 3D bioprinting has not yet been used to generate lacrimal glands, salivary gland organoids, including secretory epithelial, ductal, and, myoepithelial, neuronal compartments, have been successfully printed and exhibited over a 90% cell viability rate 3 days after differentiation [38]. Transplantation of these salivary gland-like structures into gland-irradiated mice significantly stimulated epithelial growth and innervation [38]. These results offer support for magnetic 3D bioprinting in future applications in lacrimal gland regeneration.
Role of Fibroblast Growth Factor Signaling in the Growth of Lacrimal Gland Epithelium
While previous sections focused on the delivery of stem cells and tissue engineering, another strategy involves the modulation of endogenous growth factors to stimulate lacrimal gland regeneration.
Fibroblast growth factors (FGF) are known to contribute to cell proliferation and survival, differentiation, and repair, and play an important role in embryonic development [39]. Specific FGF subtypes modulated lacrimal gland development in an explant model [40]. Addition of FGF3 to the explant culture halted lacrimal gland bud formation, while the addition of FGF7 led to extensive growth with little to no stalk region. The addition of FGF 10 contributed to the development of a well-defined distal bud with proximal stalk morphology [40]. BrdU labeling revealed that FGF7 induced cell proliferation throughout the explant, FGF10 induced proliferation preferentially in the bud area, and FGF3 yielded no significant increase in cell proliferation, suggesting that specific FGFs may be essential in proper lacrimal gland development. Additionally, FGF10 injections into the lacrimal glands of NOD mice showed increased cell proliferation compared to controls. The importance of FGF-receptor 2b in modulating lacrimal gland growth was highlighted by findings showing that mice with attenuated FGF receptor 2b signaling were unable to significantly regenerate acinar structures after lacrimal gland injury with IL-1α injection. ERK 1 and 2 – downstream factors in FGF signaling – may be important in mediating lacrimal gland growth [40].
Conclusion
The vast majority of current treatment options for dry eye disease are temporizing. Advances in lacrimal gland regeneration offer the possibility of future, more permanent options for the treatment of DED. While many of the treatment strategies discussed are still in the early stages of investigation, they provide the foundation for future studies with the goal of develo** novel, effective, and lasting modalities for the treatment of DED.
Data Availability
No datasets were generated or analysed during the current study.
References
O’Neil EC, Henderson M, Massaro-Giordano M, Bunya VY. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30(3):166–78. https://doi.org/10.1097/ICU.0000000000000569.
Hossain P, Siffel C, Joseph C, Meunier J, Markowitz JT, Dana R. Patient-reported burden of dry eye disease in the UK: a cross-sectional web-based survey. BMJ Open. 2021;11(3):e039209. https://doi.org/10.1136/bmjopen-2020-039209.
Hakim FE, Farooq AV. Dry Eye Disease: an update in 2022. JAMA. 2022;327(5):478–9. https://doi.org/10.1001/jama.2021.19963.
Drew VJ, Tseng CL, Seghatchian J, Burnouf T. Reflections on Dry Eye Syndrome Treatment: therapeutic role of Blood products. Front Med (Lausanne). 2018;5:33. https://doi.org/10.3389/fmed.2018.00033.
Liu CY, Hirayama M, Ali M, Shah D, Aakalu VK. Strategies for regenerating the lacrimal gland. Curr Ophthalmol Rep. 2017;5(3):193–8. https://doi.org/10.1007/s40135-017-0142-3.
Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, et al. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of Sjögren’s syndrome. Stem Cells Int. 2017;2017:3134543. https://doi.org/10.1155/2017/3134543.
Abughanam G, Elkashty OA, Liu Y, Bakkar MO, Tran SD. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int J Mol Sci. 2019;20(19). https://doi.org/10.3390/ijms20194750.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. https://doi.org/10.1186/1478-811X-9-12.
Yang N, Liu X, Chen X, Yu S, Yang W, Liu Y. Stem cells from exfoliated deciduous teeth transplantation ameliorates Sjögren’s syndrome by secreting soluble PD-L1. J Leukoc Biol. 2022;111(5):1043–55. https://doi.org/10.1002/JLB.6MA0921-752RR. Transplantion of stem cells harvested from human exfoliated deciduous teeth in a dry eye mouse model showed reduction of lacrimal gland inflammation and dry eye signs.
Jaffet J, Mohanty A, Veernala I, Singh S, Ali MJ, Basu S et al. Human Lacrimal Gland Derived Mesenchymal Stem Cells - Isolation, Propagation, and Characterization. Invest Ophthalmol Vis Sci. 2023;64(10):12. https://doi.org/10.1167/iovs.64.10.12. Found presence of MSC populations within human lacrimal glands, which demonstrated similar qualities as MSCs derived from bome marrow.
Hernández AE, García E. Mesenchymal stem cell therapy for Alzheimer’s Disease. Stem Cells Int. 2021;2021:7834421. https://doi.org/10.1155/2021/7834421.
Guo S, Zhang Y, Meng F, Li M, Yu Z, Chen Y, et al. Multiple intravenous injections of Valproic Acid-Induced Mesenchymal Stem Cell from Human-Induced pluripotent stem cells improved cardiac function in an Acute Myocardial Infarction Rat Model. Biomed Res Int. 2020;2020:2863501. https://doi.org/10.1155/2020/2863501.
Wang L, Huang S, Li S, Li M, Shi J, Bai W, et al. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug Des Devel Ther. 2019;13:4331–40. https://doi.org/10.2147/DDDT.S225613.
Huang W, Tourmouzis K, Perry H, Honkanen RA, Rigas B. Animal models of dry eye disease: useful, varied and evolving (review). Exp Ther Med. 2021;22(6):1394. https://doi.org/10.3892/etm.2021.10830.
Shandil RK, Dhup S, Narayanan S. Evaluation of the Therapeutic Potential of Mesenchymal Stem Cells (MSCs) in Preclinical Models of Autoimmune Diseases. Stem Cells Int. 2022;2022:6379161. doi:https://doi.org/10.1155/2022/6379161. Demonstrated efficacy of transplanting human MSCs into dry eye mouse models in reducing signs of dry eye, imflammatory foci, and pro-inflammatory cytokines.
Shin S, Yoon SG, Kim M, Cheon EJ, Jeon Y, Lee HJ, et al. The Effect of Mesenchymal Stem Cells on Dry Eye in Sjogren Syndrome Mouse Model. Int J Mol Sci. 2023;24(2). doi: https://doi.org/10.3390/ijms24021039. Demonstrated efficacy of transplanting human MSCs into dry eye mouse models in reducing signs of dry eye , imflammatory foci, and pro-inflammatory cytokines.
Kastrup J, Haack-Sørensen M, Juhl M, Harary Søndergaard R, Follin B, Drozd Lund L, et al. Cryopreserved off-the-Shelf Allogeneic adipose-derived stromal cells for therapy in patients with ischemic heart disease and heart Failure-A Safety Study. Stem Cells Transl Med. 2017;6(11):1963–71. https://doi.org/10.1002/sctm.17-0040.
Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30. https://doi.org/10.2217/rme-2018-0161.
Møller-Hansen M, Larsen AC, Toft PB, Lynggaard CD, Schwartz C, Bruunsgaard H, et al. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2021;19:43–52. https://doi.org/10.1016/j.jtos.2020.11.013.
Aluganti Narasimhulu C, Singla DK. The role of bone morphogenetic protein 7 (BMP-7) in inflammation in Heart diseases. Cells. 2020;9(2). https://doi.org/10.3390/cells9020280.
Dietrich J, Ott L, Roth M, Witt J, Geerling G, Mertsch S, et al. MSC Transplantation improves lacrimal gland regeneration after surgically Induced Dry Eye Disease in mice. Sci Rep. 2019;9(1):18299. https://doi.org/10.1038/s41598-019-54840-5.
Hu S, Di G, Cao X, Liu Y, Wang Y, Zhao H, et al. Lacrimal gland homeostasis is maintained by the. Mol Vis. 2021;27:679–90.
Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–79. https://doi.org/10.1007/s00586-008-0745-3.
Hosseinkhani M, Mehrabani D, Karimfar MH, Bakhtiyari S, Manafi A, Shirazi R. Tissue engineered scaffolds in regenerative medicine. World J Plast Surg. 2014;3(1):3–7.
Lin H, Sun G, He H, Botsford B, Li M, Elisseeff JH, et al. Three-Dimensional culture of functional adult rabbit lacrimal gland epithelial cells on Decellularized Scaffold. Tissue Eng Part A. 2016;22(1–2):65–74. https://doi.org/10.1089/ten.TEA.2015.0286.
Massie I, Spaniol K, Barbian A, Poschmann G, Stühler K, Geerling G, et al. Evaluation of Decellularized Porcine Jejunum as a Matrix for Lacrimal Gland Reconstruction in Vitro for treatment of Dry Eye Syndrome. Invest Ophthalmol Vis Sci. 2017;58(12):5564–74. https://doi.org/10.1167/iovs.16-20759.
Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–30. https://doi.org/10.1038/nmeth1012.
Hirayama M, Tsubota K, Tsuji T. Generation of a Bioengineered Lacrimal Gland by using the Organ Germ Method. Methods Mol Biol. 2017;1597:153–65. https://doi.org/10.1007/978-1-4939-6949-4_11.
Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497. https://doi.org/10.1038/ncomms3497.
Gunti S, Hoke ATK, Vu KP, London NR. Organoid and Spheroid Tumor models: techniques and applications. Cancers (Basel). 2021;13(4). https://doi.org/10.3390/cancers13040874.
Massie I, Spaniol K, Barbian A, Geerling G, Metzger M, Schrader S. Development of lacrimal gland spheroids for lacrimal gland tissue regeneration. J Tissue Eng Regen Med. 2018;12(4):e2001–9. https://doi.org/10.1002/term.2631.
Tiwari S, Nair RM, Vamadevan P, Ali MJ, Naik MN, Honavar SG, et al. Establishing and characterizing lacrispheres from human lacrimal gland for potential clinical application. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):717–27. https://doi.org/10.1007/s00417-018-3926-8.
Lin H, Liu Y, Yiu S. Three Dimensional Culture of potential epithelial progenitor cells in human lacrimal gland. Transl Vis Sci Technol. 2019;8(4):32. https://doi.org/10.1167/tvst.8.4.32.
Jeong SY, Choi WH, Jeon SG, Lee S, Park JM, Park M et al. Establishment of functional epithelial organoids from human lacrimal glands. Stem Cell Res Ther. 2021;12(1):247. doi: 10.1186/s13287-021-02133-y. Generated lacrimal gland organoids from excised human lacrimal tissue, which showed similar histological features and lacrimal gland marker expression to those of native lacrimal gland tissue.
Asal M, Koçak G, Sarı V, Reçber T, Nemutlu E, Utine CA, et al. Development of lacrimal gland organoids from iPSC derived multizonal ocular cells. Front Cell Dev Biol. 2022;10:1058846. https://doi.org/10.3389/fcell.2022.1058846. Novel method of generating lacrimal gland organoids from induced pluripotent stem cells. The organoids secreted tears that contained tear proteins found in normal healthy human lacrimal glands.
Li Z, Duan H, Li W, Hu X, Jia Y, Zhao C, et al. Rapid differentiation of Multi-zone Ocular cells from Human Induced Pluripotent Stem cells and generation of corneal epithelial and endothelial cells. Stem Cells Dev. 2019;28(7):454–63. https://doi.org/10.1089/scd.2018.0176.
Rodboon T, Yodmuang S, Chaisuparat R, Ferreira JN. Development of high-throughput lacrimal gland organoid platforms for drug discovery in dry eye disease. SLAS Discov. 2022;27(3):151–8. https://doi.org/10.1016/j.slasd.2021.11.002.
Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018;180:52–66. https://doi.org/10.1016/j.biomaterials.2018.06.011.
Farooq M, Khan AW, Kim MS, Choi S. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells. 2021;10(11). https://doi.org/10.3390/cells10113242.
Thotakura S, Basova L, Makarenkova HP. FGF gradient controls boundary position between proliferating and differentiating cells and regulates lacrimal gland Growth Dynamics. Front Genet. 2019;10:362. https://doi.org/10.3389/fgene.2019.00362.
Acknowledgements
David Murrel at the University of Michigan, Ann Arbor for creating both figures.
Author information
Authors and Affiliations
Contributions
All authors attest that they meet the current ICMJE criteria for authorship. The authors confirm their contribution to the paper as follows: Alexander C. Lieu – Conceptualization of text and figures, Writing – Original Draft and Editing Marissa K. Shoji – Conceptualization of text and figures, Validation, Writing – Reviewing and EditingVinay K. Aakalu – Conceptualization of text and figures, Validation, Writing – Reviewing and Editing, Supervision Catherine Y. Liu – Conceptualization of text and figures, Validation, Writing – Reviewing and Editing, Supervision.
Corresponding author
Ethics declarations
Catherine Y. Liu - Principal Investigator of Lassen-Sponsored clinical trial, past principal investigator of Amgen-Sponsored clinical trial, royalties from Wolters Kluwers Health
Vinay K. Aakalu -None of the following directly relate to the current manuscript: Patents: University of Illinois; University of Michigan Clinical Trials: Amgen, Sling Therapeutics, Roche.
Research Funding: National Institutes of Health (NINDS, NEI); Research to Prevent Blindness; Prior Funding: Veterans Affairs Office of Research and Development, Department of Defense, National Science Foundation.
Equity: 8th Line; ViSo Therapeutics Inc.
The remaining authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lieu, A.C., Shoji, M.K., Aakalu, V.K. et al. Approaches to Restoring Lacrimal Gland Function: From stem Cells to Tissue Engineering. Curr Ophthalmol Rep (2024). https://doi.org/10.1007/s40135-024-00326-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s40135-024-00326-1