Abstract
Introduction
In this single-center retrospective cohort study, we investigated the efficacy of letermovir in preventing Cytomegalovirus (CMV) infection in patients with aplastic anemia (AA) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods
Based on whether or not letermovir was used for preventing CMV infection, the patients were categorized into two groups: letermovir and control groups. The overall survival (OS) rate and cumulative incidence of CMV infection during the first 100 days after allo-HSCT were evaluated. The study included 21 matched pairs of patients, identified through propensity score matching analysis, to compare CMV infection rates, treatment efficacy, and regression.
Results
The incidence of CMV infection within 100 days after transplantation was significantly lower in the letermovir group than in the control group (26.5 vs. 77.4%, respectively; P < 0.001), among a total of 87 patients who underwent the transplant. In the matched cohort of 21 patients with AA, the letermovir group also showed a significantly reduced cumulative incidence of CMV infection (14.3 vs. 90.5% in the control group; P < 0.001). Compared to the control group, patients with CMV infection in the letermovir group had lower CMV-DNA load and a shorter clearance time. However, there was no significant difference in OS between both groups (P = 0.34).
Conclusions
Letermovir effectively prevents CMV infection in allo-HSCT recipients with AA and demonstrates a high safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Aplastic anemia (AA) patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) are at high risk for Cytomegalovirus (CMV) infection, which can lead to severe complications and affect the overall success of the transplantation. |
Effective prevention strategies for CMV infection post-transplantation are crucial to improve patient outcomes, but the specific efficacy and safety of letermovir in patients with AA have not been extensively studied. |
What was learned from the study? |
The study demonstrated that letermovir significantly reduced the incidence of CMV infection within the first 100 days after allo-HSCT in patients with AA. |
Letermovir showed a high safety profile, with most patients tolerating the treatment well and experiencing a reduction in CMV-DNA load and infection clearance time. |
Introduction
Aplastic anemia (AA) is a severe hematopoietic disorder characterized by rapid onset and progression. Currently, the principal intervention for AA is allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1]. Cytomegalovirus (CMV) is among the most common opportunistic infections that occur in patients post-HSCT. Early CMV reactivation can occur in 70% of patients post-HSCT during the immunocompromised phase [2, 1). In the matched group analysis (42 patients), the letermovir group showed a significantly lower incidence of CMV infection (14.3 vs. 90.5% in the control group, P < 0.001; Fig. 3). No cases of CMV disease affecting organ function were observed in the letermovir group. The median maximum peak value of CMV-DNA load was 1900 copies/ml (1070–2270 copies/ml) for letermovir patients and 3060 copies/ml (808–9910 copies/ml) for the control group (P = 0.0165; Fig. 4), indicating a higher viral load in the control group. Additionally, two control-group patients developed CMV retinitis, evidencing the severity of CMV infection in this group.
Regression of CMV Infection
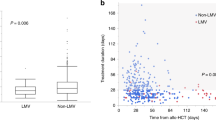
The mean duration from diagnosis to clearance of CMV infection in the letermovir and control groups was 11.7 and 18.6 days, respectively, which was significantly different (P = 0.0219; Fig. 5). Although three patients in the letermovir group were diagnosed with CMV infection, none experienced organ function impairment or involvement until the end of the follow-up. Conversely, two of 18 patients in the control group with CMV DNAemia developed CMV retinitis.
Immune Function Reconstitution within 100 days after allo-HSCT
One month after HSCT, no statistical differences were observed in the absolute number of T cell subsets (CD3+, CD4+, CD8+), CD19+B cells, CD16+CD56+NK cells, and serum immunoglobulin concentrations (IgM, IgG, IgA) levels between the letermovir and control groups. Two months after transplantation, there were higher CD3+ and CD8+ T lymphocytes in the control group, with statistical differences between both groups. Three months after transplantation, there were statistical differences in CD19+ B cells, CD16+CD56+ NK cells, and IgG in both groups, which were higher in the control group. Overall, patients in the control group had higher reconstitution of immune function after transplantation than in the letermovir group Fig. 6.
Discussion
CMV belongs to the family of human herpesviruses (HHVs), and it is also known as HHV-5. CMV remains latent in approximately 40–70% of children and 60–90% of adults [15]. CMV activation by the recipient or donor source is observed in patients after allo-HSCT, which causes direct and indirect virulence and increases the risk of bacterial and fungal infections, thereby suppressing the immune system [16].
AA presents a severe and acute condition where allo-HSCT stands as the primary treatment. Prior to transplantation, patients with AA are immunocompromised due to disease characteristics, which increases their risk of develo** various potential infections. Typically, a nonmyeloablative conditioning regimen involving immunosuppressive agents is employed in AA transplantation. A prolonged post-transplantation administration of immunosuppressive drugs is compulsory to prevent rejection and treat GVHD, leading to a slower re-establishment of immune function and a relatively higher rate of CMV infection than in patients with hematologic malignancies after transplantation [6, 7]. In our center, the incidence of CMV infection in patients with AA was 84.8% after 100 days of transplantation before using letermovir as a prophylactic agent. Furthermore, CMV infections are more prone in substitute donor transplants than MSD-HSCT, and the median time onset of CMV infection is 30 days after allo-HSCT. Consequently, our center initiated CMV prophylaxis with letermovir from the day of neutrophil engraftment following transplantation. As CMV activation and CMV disease-related morbidity were found to be associated with transplantation-related mortality, preventing, detecting, and treating CMV infection is crucial, especially during the early stage after transplantation [26]. However, the overall mortality rate of patients in both groups was not significantly different, which may be attributed to the small sample size and short follow-up duration. Using letermovir as a primary prophylaxis in patients with AA after allo-HSCT significantly reduces CMV infection and demonstrates a high safety profile. Therefore, letermovir is currently used as a prophylactic for patients with AA undergoing allo-HSCT in our institution.
At present, primary prophylaxis with letermovir within 100 days after transplantation is the standard protocol for CMV-seropositive allo-HSCT recipients, as endorsed by the latest CMV prophylaxis guidelines of The American Society of Transplant and Cellular Therapy [27]. Letermovir discontinuation significantly increased the cumulative incidences of CMV infection within 200 days post-transplantation. As this study utilized letermovir for a short period, CMV infection occurring only within 100 days after transplantation was analyzed. The incidence of CMV infection in patients beyond the 100-day timeframe is still being monitored. Therefore, the timing of drug discontinuation should be further explored. Ongoing randomized clinical trials have extended the duration of prophylaxis in high-risk patients to 200 days post-transplantation, wherein the effects and benefits should be further explored. In this study, we have not explored the efficacy of letermovir therapy for treating pre-existing CMV infection and resistance to letermovir. A study has shown a low success rate for treating patients with pre-existing CMV activation using letermovir [23]. Studies investigating the use of letermovir for treating CMV infection after transplantation are limited. Here, we treated a few patients with letermovir, but they were excluded from this study due to the small sample size and short follow-up duration.
Although the patients’ overall tolerability to letermovir was good, the possibility of other adverse events exists due to the complex drug interactions [6]. In our center, patients were not prevented from letermovir for a long time, and the follow-up duration was short. Therefore, the long-term effects and cumulative incidences of CMV infection must be monitored continuously. In addition, the viral load of patients in the letermovir group at the time of CMV infection was lower compared to the control group. The treatment duration of patients in the letermovir group with intravenous antiviral drugs was short. Treating CMV infection poses a significant economic burden on patients. Hence, further pharmacoeconomic analyses comparing preventive strategies in transplant populations are essential to determine the role of letermovir in CMV management [24].
Our study has one limitation, which is its small sample size. Therefore, future large sample-sized studies exploring the effect of letermovir in preventing CMV infection after HSCT are warranted.
Conclusions
CMV infection is a dangerous complication in patients with AA undergoing allo-HSCT. Researchers have attempted to balance the risk of CMV infection and the toxicity of therapeutic agents, considering the adverse effects and resistance to previous nucleoside analogs. Letermovir offers a new option for managing CMV infection in patients post-HSCT due to its unique mechanism of action. In addition, it has demonstrated a high safety profile and effectiveness in preventing and treating CMV infection in patients with AA post-transplantation, especially in patients with slow reestablishment of immune function.
Availability of Data and Materials
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70.
Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–58.
Green ML, Leisenring W, **e H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–27.
Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–38.
Xu LP, ** S, Wang SQ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10(1):25.
Saullo JL, Miller RA. Cytomegalovirus therapy: role of letermovir in prophylaxis and treatment in transplant recipients. Annu Rev Med. 2023;74:89–105.
Mo W, Chen X, Zhang X, et al. The potential association of delayed t lymphocyte reconstitution within six months post-transplantation with the risk of cytomegalovirus retinitis in severe aplastic anemia recipients. Front Cell Infect Microbiol. 2022;12:900154.
Green ML, Leisenring WM, **e H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–24.
Zhang Y, Wu L, Mo W, et al. Comparable outcomes of first-line hematopoietic stem cell transplantation from unrelated and matched sibling donors in adult patients with aplastic anemia: a retrospective single-center study. Biol Blood Marrow Transplant. 2019;25(8):1567–75.
Zhang YY, Mo WJ, Zuo YY, et al. Comparable survival outcome between transplantation from haploidentical donor and matched related donor or unrelated donor for severe aplastic anemia patients aged 40 years and older: A retrospective multicenter cohort study. Clin Transplant. 2020;34(3): e13810.
Blyth E, Withers B, Clancy L, et al. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence. 2016;7(8):967–80.
Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433–44.
Verghese PS, Schleiss MR. Letermovir treatment of human cytomegalovirus infection antiinfective agent. Drugs Future. 2013;38(5):291–8.
Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–7.
Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87–91.
Powers C, Defilippis V, Malouli D, et al. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–59.
Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59(4):473–81.
Styczyński J, Czyżewski K, Dębski R. Primary prophylaxis with letermovir for prevention of CMV infection in two children. Acta Haematol Pol. 2020;51(4):263–4.
Chiereghin A, Belotti T, Borgatti EC, et al. Off-label use of letermovir as preemptive anti-cytomegalovirus therapy in a pediatric allogeneic peripheral blood stem cell transplant. Infect Drug Resist. 2021;14:1185–90.
Richert-Przygonska M, Jaremek K, Debski R, et al. Letermovir prophylaxis for cytomegalovirus infection in children after hematopoietic cell transplantation. Anticancer Res. 2022;42(7):3607–12.
Daukshus NP, Cirincione A, Siver M, et al. Letermovir for cytomegalovirus prevention in adolescent patients following hematopoietic cell transplantation. J Pediatric Infect Dis Soc. 2022;11(7):337–40.
Mori Y, **nouchi F, Takenaka K, et al. Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transplant. 2021;56(4):853–62.
Anderson A, Raja M, Vazquez N, et al. Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transplant. 2020;34(7): e13866.
Derigs P, Radujkovic A, Schubert ML, et al. Letermovir prophylaxis is effective in preventing cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation: single-center real-world data. Ann Hematol. 2021;100(8):2087–93.
Wang LL, Mo WJ, Zhang YP, et al. Clinical analysis of cmv infection after allogeneic hematopoietic stem cell transplantation in severe aplastic anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(3):944–50.
Zamora D, Duke ER, **e H, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138(1):34–43.
Hakki M, Aitken SL, Danziger-Isakov L, et al. American society for transplantation and cellular therapy series: #3-prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(9):707–19.
Funding
This study was supported by grants from the Innovative Clinical Technique of Guangzhou (No. 2019GX04) (YPZ), 2019 Annual Research Project of The China Marrow Donor Program (No. CMDP201902) (SQW), Guang Zhou Basic and Applied Basic Research Foundation (202201010062) (CXW), Guangzhou Municipal Science and Technology project (202002030035) (SQW), Natural Science Foundation of Guangdong Province (2018A030130179) (MZ) and Science and Technology Projects in Guangzhou (202201010062) (XWC). The journal’s rapid service fee was funded by Science and Technology Projects in Guangzhou (202201010062) (XWC).
Author information
Contributions
WJM, and SQW: Contributed to the concept development and study design; coordinated the research and helped to write the manuscript. YLZ and XWC: Collected the clinical information, analyzed the data, and wrote the manuscript. MZ, YPZ, CTC, RQZ, YML, FFY, SLX, CXW, WZ, TFD, and SYP: Diagnosed and treated the patients, provided clinical information, and contributed to the follow-up of patients. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Guangzhou First People’s Hospital and conducted in accordance with the principles of the Declaration of Helsinki.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, Y., Chen, X., Zhou, M. et al. Letermovir Effectively Prevents Cytomegalovirus Infection in Patients with Aplastic Anemia After Hematopoietic Stem Cell Transplantation: A Real-World Retrospective Cohort Study. Infect Dis Ther 13, 345–359 (2024). https://doi.org/10.1007/s40121-024-00917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00917-2