Abstract
Introduction
With COVID-19 vaccination rolled out globally, increasing numbers of studies have shown that booster vaccines can enhance an individual’s protection against the infection, hospitalization, and death caused by SARS-CoV-2. This study evaluated the effectiveness of COVID-19 vaccine BBIBP-CorV booster against being infected (susceptibility), infecting others (infectiousness), and spreading the disease from one to another (transmission).
Methods
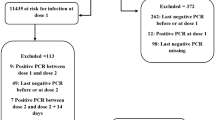
This retrospective cohort study investigated the close contacts of all officially ascertained COVID-19 confirmed cases in Urumqi, China between August 1 and September 7, 2022. Eligible records were divided into four subcohorts based on the vaccination status of both the close contact and their source case: group 2-2, 2-dose contacts seeded by 2-dose source case (as the reference level); group 2-3, 3-dose contacts seeded by 2-dose source case; group 3-2, 2-dose contacts seeded by 3-dose source case; and group 3-3, 3-dose contacts seeded by 3-dose source case. In the four subcohorts, multivariate logistic regression models were used to examine the vaccine effectiveness (VE) for the BBIBP-CorV booster dose. We adjusted for potential confounding variables, including the sex and age of source cases and close contacts, the calendar week of contact history and contact settings. We evaluated the statistical uncertainty using a 95% confidence interval (CI). In addition, we conducted subgroup analyses to evaluate VE by sex.
Results
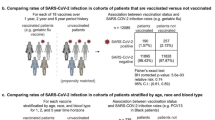
The sample sizes of groups 2-2, 2-3, 3-2, and 3-3 were 1184, 3773, 4723, and 27,136 individuals, respectively. Overall VE against susceptibility (group 2-3 vs 2-2) was 42.1% (95% CI 10.6, 62.5), VE against infectiousness (group 3-2 vs 2-2) was 62.0% (95% CI 37.2, 77.0), and VE against transmission (group 3-3 vs 2-2) was 83.7% (95% CI 75.1, 89.4). In the sex-stratified subgroups, male close contacts showed similar VE compared to the overall. However, among female close contacts, while the booster dose improved VE against infectiousness and VE against susceptibility, the VEs were not significantly different from zero.
Conclusion
BBIBP-CorV vaccine booster was associated with mild to moderate levels of protection against Omicron susceptibility, infectiousness, and transmission. Real-world assessment of protective performance of COVID-19 vaccines against the risk of Omicron strains is continuously needed, and may provide information that helps vaccination strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The vaccines conferred multiple protection against infectious diseases. |
By using contact tracing data, we evaluated the vaccine effectiveness of BBIBP-CorV booster against the susceptibility, infectiousness, and transmission of Omicron strains. |
What was learned from the study? |
BBIBP-CorV vaccine booster was associated with mild to moderate levels of protection against Omicron’s susceptibility, infectiousness, and transmission. |
Real-world assessment of vaccine performance against the risk of emerging SARS-CoV-2 genetic variants is continuously needed. |
Introduction
Since the outbreak of the COVID-19, vaccination has been regarded as one of the most effective measures to combat the disease [1,2,3]. Vaccine developers and manufacturers have conducted large-scale clinical trials worldwide and reported corresponding vaccine efficacy results [Full size image
The overall VE were shown in Table 2. After adjustment for potential confounding variables, the VE against infectiousness was 62.0% (95% CI 37.2–77.0), the VE against susceptibility was 42.1% (95% CI 10.6–62.5), and the VE against transmission was 83.7% (95% CI 75.1–89.4). In the subgroup analyses, male close contacts showed similar VE compared to the overall sample. However, among female close contacts, while the booster dose improved the VE against infectiousness and VE against susceptibility, the VE estimations were not significantly different from zero.