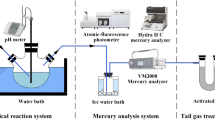
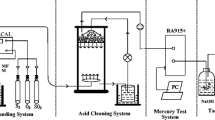
Abstract
Experiments were conducted in a simulated wet flue gas desulfurization (WFGD) system to investigate the effects of sulfite, slurry temperature, pH value and atmosphere on mercury migration and re-emission. The mechanisms were discussed based on the kinetics and chemical equilibrium. The results indicate that SO32− is a key factor for Hg0 re-emission in WFGD system. Increasing SO32− concentration at a relatively low level can promote Hg2+ in liquid phase reducing to gaseous Hg0, whereas Hg0 re-emission is significantly inhibited with excess SO32−. The reaction rate of mercury reduction is calculated to increase by approximately ten times when the slurry temperature rises from 40 to 60 °C. It reveals that a higher slurry temperature can accelerate mercury reduction. The proportion of mercury in liquid phase is detected to decline sharply with a drop in pH value. The mechanisms were further proposed that SO32− tends to be protonated and the stabilities of mercury–sulfite complexes are weakened at low pH value. Under both N2/O2 and O2/CO2 atmospheres, increasing O2 concentration can suppress Hg0 re-emission. However, the variation in CO2 concentrations has no impact on Hg0 re-emission. Compared with O2/CO2 atmosphere, there is more Hg0 re-emission under N2/O2 atmosphere due to the secondary release of Hg0.













Similar content being viewed by others
References
Acuña-Caro C, Brechtel K, Scheffknecht G, Braß M (2009) The effect of chlorine and oxygen concentrations on the removal of mercury at an FGD-batch reactor. Fuel 88(12):2489–2494
Bilinski H, Markovic M, Gessner M (1980) Solubility and equilibrium constants of mercury (ii) in carbonate solutions (25 °C, i = 0.5 mol dm−3). Inorg Chem 19(11):3440–3443
Chang R (2005) Physical chemistry for the biosciences. University Science Books, Mill Valley
Chang JC, Ghorishi SB (2003) Simulation and evaluation of elemental mercury concentration increase in flue gas across a wet scrubber. Environ Sci Technol 37(24):5763–5766
Chang L, Zhao Y, Li H, Tian C, Zhang Y, Yu X, Zhang J (2017) Effect of sulfite on divalent mercury reduction and re-emission in a simulated desulfurization aqueous solution. Fuel Process Technol 165:138–144
Cheng X, Bi XT (2014) A review of recent advances in selective catalytic nox reduction reactor technologies. Particuology 16:1–18
Clever HL, Johnson SA, Derrick ME (1985) The solubility of mercury and some sparingly soluble mercury salts in water and aqueous electrolyte solutions. J Phys Chem Ref Data 14(3):631–680
Díaz-Somoano M, Unterberger S, Hein KR (2005) Using wet-FGD systems for mercury removal. J Environ Monit 7(9):906–909
Dίaz-Somoano M, Unterberger S, Hein KRG (2007) Mercury emission control in coal-fired plants: the role of wet scrubbers. Fuel Process Technol 88(3):259–263
Elder JF (1975) Complexation side reactions involving trace metals in natural water systems. I. Limnol Oceanogr 20(1):96–102
Evans MG, Polanyi M (1935) Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans Faraday Soc 31:875–894
Feinberg AI, Kurien U, Ariya PA (2015) The kinetics of aqueous mercury (ii) reduction by sulfite over an array of environmental conditions. Water Air Soil Pollut 226(4):119
Fernandez-Miranda N, Lopez-Anton MA, Torre-Santos T, Diaz-Somoano M, Martinez-Tarazona MR (2016) Impact of oxy-fuel conditions on elemental mercury re-emission in wet flue gas desulfurization systems. Environ Sci Technol 50(13):7247–7253
Galbreath KC, Zygarlicke CJ (2000) Mercury transformations in coal combustion flue gas. Fuel Process Technol 65:289–310
Habib M, Badr H, Ahmed S, Ben-Mansour R, Mezghani K, Imashuku S, la O’ G, Shao-Horn Y, Mancini N, Mitsos A (2011) A review of recent developments in carbon capture utilizing oxy-fuel combustion in conventional and ion transport membrane systems. Int J Energy Res 35(9):741–764
Heidel B, Hilber M, Scheffknecht G (2014) Impact of additives for enhanced sulfur dioxide removal on re-emissions of mercury in wet flue gas desulfurization. Appl Energy 114:485–491
Ho T-L (2012) Hard and soft acids and bases principle in organic chemistry. Elsevier, Amsterdam
Huang W, Qu Z, Chen W, Xu H, Yan N (2016) An enhancement method for the elemental mercury removal from coal-fired flue gas based on novel discharge activation reactor. Fuel 171:59–64
Jiang YZ, Chen CM, Jiang LX, Liu ST, Wang B (2012) Study of mercury re-emission from simulated wet flue gas desulfurization liquors. Adv Mater Res 610–613:2033–2037
Liu Y, Li Y, Xu H, Xu J (2019) Oxidation removal of gaseous Hg0 using enhanced-fenton system in a bubble column reactor. Fuel 246:358–364
Mackey TK, Contreras JT, Liang BA (2014) The minamata convention on mercury: attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci Total Environ 472:125–129
Marsicano F, Hancock RD (1976) A potentiometric and calorimetric study of the thermodynamics of formation of some of the complexes of the d10 metal ions silver (i), mercury (ii), and cadmium (ii) with thiodiglycol, thiourea, and the sulphite ion. J Coord Chem 6(1):21–29
Munthe J, **ao Z, Lindqvist O (1991) The aqueous reduction of divalent mercury by sulfite. Water Air Soil Pollut 56(1):621–630
Naik MM, Dubey S (2017) Lead-and mercury-resistant marine bacteria and their application in lead and mercury bioremediation. In: Marine pollution and microbial remediation. Springer, Berlin, pp 29–40
Ochoa-Gonzalez R, Diaz-Somoano M, Martinez-Tarazona MR (2014) A comprehensive evaluation of the influence of air combustion and oxy-fuel combustion flue gas constituents on Hg(0) re-emission in WFGD systems. J Hazard Mater 276:157–163
Omine N, Romero CE, Kikkawa H, Wu S, Eswaran S (2012) Study of elemental mercury re-emission in a simulated wet scrubber. Fuel 91(1):93–101
Outridge PM, Mason R, Wang F, Guerrero S, Heimbürger-Boavida L (2018) Updated global and oceanic mercury budgets for the united nations global mercury assessment 2018. Environ Sci Technol 52(20):11466–11477
Pearson RG (1968) Hard and soft acids and bases, HSAB, part ii: underlying theories. J Chem Educ 45(10):643
Peng B, Liu Z, Chai L, Liu H, Yang S, Yang B, **ang K, Liu C (2017) Effect of copper ions on the mercury re-emission in a simulated wet scrubber. Fuel 190:379–385
Solis KL, Nam G-U, Hong Y (2017) Mercury (ii) reduction and sulfite oxidation in aqueous systems: kinetics study and speciation modeling. Environ Chem 14(3):151–159
Stergaršek A, Horvat M, Kotnik J, Tratnik J, Frkal P, Kocman D, Jaćimović R, Fajon V, Ponikvar M, Hrastel I (2008) The role of flue gas desulphurisation in mercury speciation and distribution in a lignite burning power plant. Fuel 87(17–18):3504–3512
Stolle R, Koeser H, Gutberlet H (2014) Oxidation and reduction of mercury by SCR denox catalysts under flue gas conditions in coal fired power plants. Appl Catal B 144:486–497
Sun M, Lou Z, Cheng G, Baig SA, Fang L, Zhou X, Shen Y, Xu X (2015) Process migration and transformation of mercury in simulated wet flue gas desulfurization slurry system. Fuel 140:136–142
Tip** E (2007) Modelling the interactions of Hg (ii) and methylmercury with humic substances using wham/model vi. Appl Geochem 22(8):1624–1635
Van Loon L, Mader E, Scott SL (2000) Reduction of the aqueous mercuric ion by sulfite: UV spectrum of HgSO3 and its intramolecular redox reaction. J Phys Chem A 104(8):1621–1626
Van Loon LL, Mader EA, Scott SL (2001) Sulfite stabilization and reduction of the aqueous mercuric ion: kinetic determination of sequential formation constants. J Phys Chem A 105(13):3190–3195
Wall TF (2007) Combustion processes for carbon capture. Proc Combust Inst 31(1):31–47
Wang Y, Liu Y, Wu Z, Mo J, Cheng B (2010) Experimental study on the absorption behaviors of gas phase bivalent mercury in Ca-based wet flue gas desulfurization slurry system. J Hazard Mater 183(1–3):902–907
Wo J, Zhang M, Cheng X, Zhong X, Xu J, Xu X (2009) Hg2+ reduction and re-emission from simulated wet flue gas desulfurization liquors. J Hazard Mater 172(2–3):1106–1110
Wu C-L, Cao Y, He C-C, Dong Z-B, Pan W-P (2010) Study of elemental mercury re-emission through a lab-scale simulated scrubber. Fuel 89(8):2072–2080
Wu H, Chen H, Wang Q, Yang H (2019a) Characteristics and inhibition of mercury re-emission from desulfurization slurry by Fenton reagent. Fuel Process Technol 188:89–97
Wu H, Sun J, Zhou C, Yang H (2019b) Effect of additives on stabilization and inhibition of mercury re-emission in simulated desulphurization slurry. Int J Environ Sci Technol 16(12):7705–7714
Yang J, Ma S, Zhao Y, Zhang J, Liu Z, Zhang S, Zhang Y, Liu Y, Feng Y, Xu K (2017) Mercury emission and speciation in fly ash from a 35 MWth large pilot boiler of oxyfuel combustion with different flue gas recycle. Fuel 195:174–181
Zhao S, Duan Y, Lu J, Gupta R, Pudasainee D, Liu S, Liu M, Lu J (2018) Thermal stability, chemical speciation and leaching characteristics of hazardous trace elements in FGD gypsum from coal-fired power plants. Fuel 231:94–100
Acknowledgments
The authors thank the National Natural Science Foundation of China (51506099, 51709191, 51606130), the Fundamental Research Funds for the Central Universities (YJ201659) and the State Scholarship Fund from China Scholarship Council (201906240029) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: U.W. Tang.
Rights and permissions
About this article
Cite this article
Xu, J., Bao, J., Liu, H. et al. Mercury migration and re-emission in a simulated wet flue gas desulfurization system. Int. J. Environ. Sci. Technol. 18, 691–702 (2021). https://doi.org/10.1007/s13762-020-02853-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02853-3