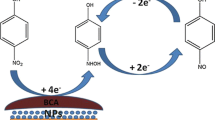
Abstract
The critical role of ferric ions in biological system and health impact posed to humans due to related toxicity caused by the consumption of iron-contaminated drinking water or food, as well as exposure to the other environmental sector, needs a sensitive method for the determination of these ions in various domains such us medical uses, biological and environmental samples. Herein, a novel impedimetric sensor based on the bases Schiff molecule, (E)-2-((phenylimino)methyl) phenol (E-PNMP), was investigated for the quantification of ferric ions. The ability to recognize ferric ions with E-PNMP was characterized by UV–Vis, which indicated that we obtained a complex E-PNMP/Fe3+ with a stoichiometry (1:2). The E-PNMP-modified electrode was characterized by electrochemical impedance spectroscopy (EIS). Under the optimal conditions, the proposed impedimetric sensor exhibits a limit of detection of 2.49 × 10–12 M in the range of concentration from 10–12 to 10–5 M. Thus, the investigated impedimetric sensor showed high sensibility, selectivity, reproducibility, and repeatability. A Pt/E-PNMP electrode was successfully applied for the determination of iron (III) in a real sample. Furthermore, to better understand the sensing mechanism of E-PNMP/iron, a computational study with DFT was conducted using the B3LYP functional and the 6311++G (d, p) basis set. The theoretical studies confirmed the formation of the complex Fe3+: E-PNMP 2 with high stability.














Similar content being viewed by others
References
X. Pang et al., Visual recognition of aliphatic and aromatic amines using a fluorescent gel: application of a sonication-triggered organogel. ACS Appl. Mater. Interfaces (2015). https://doi.org/10.1021/acsami.5b03000
V.A. Online, Luminescent Cd (II)—organic frameworks with and antibiotics † (2017). https://doi.org/10.1039/C7TA03849F
M.W. Hentze, M.U. Muckenthaler, B. Galy, C. Camaschella, Review Two to tango : regulation of mammalian iron metabolism. Cell 142, 24–38 (2010). https://doi.org/10.1016/j.cell.2010.06.028
X. Liu, E.C. Theil, Ferritins : dynamic management of biological iron and oxygen chemistry. Acc. Chem. Res. 38(3), 167–175 (2005)
F. Sefid, A. Alagheband, B. Maryam, D. Robab, N. Zahra, In silico analysis for determination and validation of iron-regulated protein from Escherichia coli. Int. J. Pept. Res. Ther. 25(4), 1523–1537 (2019). https://doi.org/10.1007/s10989-018-9797-3
L. Yan, A colorimetric and fluorescent probe based on rhodamine B for detection of Fe3+ and Cu2+ Ions. J Fluoresc 29, 1221–1226 (2019)
F. Busti, G. Marchi, S. Ugolini, A. Castagna, D. Girelli, Anemia and iron deficiency in cancer patients : role of iron replacement therapy. Pharmaceuticals (2018). https://doi.org/10.3390/ph11040094
V.C. Chang, M. Cotterchio, E. Khoo, Iron intake, body iron status, and risk of breast cancer : a systematic review and meta-analysis. BMC Cancer (2019). https://doi.org/10.1186/s12885-019-5642-0
L. Du et al., Increased iron deposition on brain quantitative susceptibility map** correlates with decreased cognitive function in Alzheimer ’ s disease. ACS Chem. Neurosci. 9, 1849–1857 (2018). https://doi.org/10.1021/acschemneuro.8b00194
Z. Liu, H. Shen, T. Lian, L. Mao, S. Tang, L. Sun, Iron deposition in substantia nigra: abnormal iron metabolism, neuroinflammatory mechanism and clinical relevance. Sci. Rep. (2017). https://doi.org/10.1038/s41598-017-14721-1
K.-M. Vuori, Aquatic ecotoxicology-study of freshwater systems stressed by toxic chemical (1995)
J. De Jong, N. Mattielli, High-accuracy determination of iron in seawater by isotope dilution multiple collector inductively coupled plasma mass spectrometry (ID-MC-ICP-MS) using nitrilotriacetic acid chelating resin for pre-concentration and matrix separation. Anal. Chim. Acta. 3, 126–139 (2008). https://doi.org/10.1016/j.aca.2008.06.013
W. Ruengsitagoon, Reverse flow injection spectrophotometric determination of iron (III) using chlortetracycline reagent. Talanta 74, 1236–1241 (2008). https://doi.org/10.1016/j.talanta.2007.08.031
B. Peng, Y. Shen, Z. Gao, M. Zhou, Y. Ma, S. Zhao, Determination of total iron in water and foods by dispersive liquid-liquid microextraction coupled with microvolume UV-vis spectrophotometry. Food Chem (2014). https://doi.org/10.1016/j.foodchem.2014.12.084
Y. Ogasawara, K. Ishii, T. Togawa, S. Tanabet, Determination of trace amounts of sulphide in human red blood cells by high-performance liquid chromatography with fluorimetric detection after derivatization With pphenylenediamine and iron(iii). Analyst 116, 1359–1363 (1991)
H. Sohrabi, A. Khataee, S. Ghasemzadeh, M.R. Majidi, Y. Orooji, Layer double hydroxides (LDHs)- based electrochemical and optical sensing assessments for quantification and identification of heavy metals in water and environment samples: a review of status and prospects. Trends Environ. Anal. Chem. (2021). https://doi.org/10.1016/j.teac.2021.e00139
É.N. Oiye et al., Electrochemical sensors containing schiff bases and their transition metal complexes to detect analytes of forensic, pharmaceutical and environmental interest. A review. Crit. Rev. Anal. Chem. 49(6), 488–509 (2019). https://doi.org/10.1080/10408347.2018.1561242
J.M.S. Alshawi, M.Q. Mohammed, H.F. Alesary, H.K. Ismail, S. Barton, Voltammetric Determination of Hg2+, Zn2+, and Pb2+ ions using a PEDOT/NTA-modified electrode. ACS Omega (2022). https://doi.org/10.1021/ACSOMEGA.2C02682/ASSET/IMAGES/LARGE/AO2C02682_0016.JPEG
E. Katz, I. Willner, Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 15(11), 913–947 (2003). https://doi.org/10.1002/ELAN.200390114
E.B. Bahadir, M.K. Sezgintürk, A review on impedimetric biosensors. Artif. Cells Nanomedicine Biotechnol. 44(1), 248–262 (2014). https://doi.org/10.3109/21691401.2014.942456
B. Szemenyei et al., When crown ethers finally click: novel, click-assembled, fluorescent enantiopure pyridino-crown ether-based chemosensors—and an N-2-aryl-1,2,3-triazole containing one. New J. Chem. (2021). https://doi.org/10.1039/d1nj04173h
L. Zhao et al., A porphyrin-based optical sensor membrane prepared by electrostatic self-assembled technique for online detection of cadmium(II). Chemosphere (2020). https://doi.org/10.1016/j.chemosphere.2019.124552
L. Eddaif, A. Shaban, J. Telegdi, Sensitive detection of heavy metals ions based on the calixarene derivatives-modified piezoelectric resonators: a review. Int. J. Environ. Anal. Chem. 99(9), 824–853 (2019). https://doi.org/10.1080/03067319.2019.1616708
S.M. Saleh, R. Ali, I.A.I. Ali, A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu(II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 183, 225–231 (2017). https://doi.org/10.1016/j.saa.2017.04.019
S. Lee et al., Iron detection and remediation with a functionalized porous polymer applied to environmental water samples. Chem. Sci. 10(27), 6651–6660 (2019). https://doi.org/10.1039/C9SC01441A
R.M. Irfan et al., Synthesis of new cadmium(II) complexes of Schiff bases as alkaline phosphatase inhibitors and their antimicrobial activity. Arab. J. Chem. (2021). https://doi.org/10.1016/j.arabjc.2021.103308
M.Q. Mohammed, H.K. Ismail, H.F. Alesary, S. Barton, Use of a Schiff base-modified conducting polymer electrode for electrochemical assay of Cd(II) and Pb(II) ions by square wave voltammetry. Chem. Pap. 76(2), 715–729 (2022). https://doi.org/10.1007/S11696-021-01882-7/TABLES/5
J. Fan et al., A polyethylenimine/salicylaldehyde modified cellulose Schiff base for selective and sensitive Fe3+ detection. Carbohydr. Polym. 228(September), 2020 (2019). https://doi.org/10.1016/j.carbpol.2019.115379
R. Patil et al., Ratiometric fluorescent scaffold giving discrete response towards iodide ion: a combined experimental and DFT study. J. Mol. Recognit. 27(11), 683–688 (2014). https://doi.org/10.1002/jmr.2392
A. Lealem, I. Mohiuddin, A. Kumar, J. Singh, V. Kumar, K. Kim, A review of the applications of Schiff bases as optical chemical sensors. Trends Anal. Chem. 116, 74–91 (2019). https://doi.org/10.1016/j.trac.2019.04.025
S. Agren, M. Chaabene, A.R. Allouche, R. BenChaâbane, M. Lahcinie, M.H.V. Baouab, Blue highly fluorescent boranil derived from anil ligand: synthesis, characterization, experimental and theoretical evaluation of solvent effect on structures and photophysical properties. Appl. Organomet. Chem. 34(9), 1–15 (2020). https://doi.org/10.1002/aoc.5764
J.W. Jebaraj, C. Balakrishnan, Biocompatible alkyne arms containing Schiff base fluorescence indicator for dual detection of Cd II and Pb II at physiological pH and its application to live cell imaging. Analyst 145, 4576–4586 (2020). https://doi.org/10.1039/d0an00862a
D. Brian, E. Benjamin, P.S. Solidi, D. Brian, E. Benjamin, P.S. Solidi, Evaluation of the tauc method for optical absorption edge determination: ZnO thin films as a model system. Physica Status Solidi 252(8), 1700–1710 (2015). https://doi.org/10.7282/T3W097T7
M. Echabaane, A. Rouis, I. Bonnamour, H. ben Ouada, Studies of aluminum (III) ion-selective optical sensor based on a chromogenic calix [4 ] arene derivative. SAA 115, 269–274 (2013). https://doi.org/10.1016/j.saa.2013.06.053
D. Najlaoui, M. Echabaane, A. ben Khélifa, A. Rouis, H. ben Ouada, Photoelectrochemical impedance spectroscopy sensor for cloxacillin based on tetrabutylammonium octamolybdate. J. Solid State Electrochem. 23(12), 3329–3341 (2019). https://doi.org/10.1007/S10008-019-04440-0/FIGURES/10
A. Rouis, M. Echabaane, N. Sakly, I. Dumazet-Bonnamour, H. BenOuada, Electrochemical analysis of a PPV derivative thin film doped with ß-ketoimine calix[4]arene in the dark and under illumination for the detection of Hg2+ ions. Synth. Met. 164, 78–87 (2013). https://doi.org/10.1016/J.SYNTHMET.2013.01.005
F. Zina, N.M. Nooredeen, S. Azzouzi, M. ben Ali, M.N. Abbas, A. Errachid, Novel sensitive impedimetric microsensor for phosphate detection based on a novel copper phthalocyanine derivative. Anal. Lett. 51(3), 371–386 (2018). https://doi.org/10.1080/00032719.2017.1322096
P. Hashemi, A. Afkhami, H. Bagheri, S. Amidi, T. Madrakian, Fabrication of a novel impedimetric sensor based on L-Cysteine/Cu(II) modified gold electrode for sensitive determination of ampyra. Anal. Chim. Acta 984, 185–192 (2017). https://doi.org/10.1016/j.aca.2017.06.038
M. Echabaane, S. Hfaiedh, B. Smiri, F. Saidi, C. Dridi, Development of an impedimetric sensor based on carbon dots and chitosan nanocomposite modified electrode for Cu(II) detection in water. J. Solid State Electrochem. 25, 1797–1806 (2021). https://doi.org/10.1007/s10008-021-04949-3
S. Kaur, I. Kaur, Self-assembly of p-aminothiophenol on gold surface: application for impedimetric and potentiometric sensing of cobalt (II) ions—a comparative study. Electroanalysis 31(12), 2507–2517 (2019). https://doi.org/10.1002/elan.201900187
H.F. Alesary, H.K. Ismail, M.Q. Mohammed, H.N. Mohammed, Z.K. Abbas, S. Barton, A comparative study of the effect of organic dopant ions on the electrochemical and chemical synthesis of the conducting polymers polyaniline, poly(o-toluidine) and poly(o-methoxyaniline). Chem. Pap. 75(10), 5087–5101 (2021). https://doi.org/10.1007/S11696-020-01477-8/TABLES/4
H. Touzi, Y. Chevalier, F. Bessueille, H. BenOuada, N. Jaffrezic-Renault, Detection of dyestuffs with an impedimetric sensor based on Cu2+-methyl-naphthyl cyclen complex functionalized gold electrodes. Sens. Actuators B Chem. 273, 1211–1221 (2018). https://doi.org/10.1016/j.snb.2018.07.011
C. Verma, L.O. Olasunkanmi, T.W. Quadri, E.S.M. Sherif, E.E. Ebenso, Gravimetric, electrochemical, surface morphology, DFT, and Monte Carlo simulation studies on three N-substituted 2-aminopyridine derivatives as corrosion inhibitors of mild steel in acidic medium. J. Phys. Chem. C 122(22), 11870–11882 (2018). https://doi.org/10.1021/acs.jpcc.8b02740
C. Verma, J. Haque, E.E. Ebenso, M.A. Quraishi, Melamine derivatives as effective corrosion inhibitors for mild steel in acidic solution: chemical, electrochemical, surface and DFT studies. Results Phys. 9, 100–112 (2018). https://doi.org/10.1016/j.rinp.2018.02.018
C. Verma, I.B. Obot, I. Bahadur, E.S.M. Sherif, E.E. Ebenso, Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies. Appl. Surf. Sci. 457, 134–149 (2018). https://doi.org/10.1016/j.apsusc.2018.06.035
X. **e et al., Study of heterogeneous electron transfer on the graphene/self-assembled monolayer modified gold electrode by electrochemical approaches. J. Phys. Chem. C 114(33), 14243–14250 (2010). https://doi.org/10.1021/jp102446w
K. Mahato, B. Purohit, K. Bhardwaj, A. Jaiswal, P. Chandra, Novel electrochemical biosensor for serotonin detection based on gold nanorattles decorated reduced graphene oxide in biological fluids and in vitro model. Biosens. Bioelectron. (2019). https://doi.org/10.1016/j.bios.2019.111502
S. Kaur, B.A. Shiekh, D. Kaur, I. Kaur, Highly sensitive sensing of Fe(III) harnessing Schiff based ionophore: an electrochemical approach supported with spectroscopic and DFT studies. J. Mol. Liq. (2021). https://doi.org/10.1016/j.molliq.2021.115954
S.F. Chin, S.C. Tan, S.C. Pang, S.M. Ng, Nitrogen doped carbon nanodots as fluorescent probes for selective detection and quantification of Ferric(III) ions. Opt. Mater. (Amst) 73, 77–82 (2017). https://doi.org/10.1016/j.optmat.2017.08.006
V.D. Doan et al., Efficient and fast degradation of 4-nitrophenol and detection of Fe(III) ions by Poria cocos extract stabilized silver nanoparticles. Chemosphere (2022). https://doi.org/10.1016/j.chemosphere.2021.131894
D.M. Arvapalli, A.T. Sheardy, K.C. Alapati, J. Wei, High quantum yield fluorescent carbon nanodots for detection of Fe (III) Ions and electrochemical study of quenching mechanism. Talanta (2020). https://doi.org/10.1016/j.talanta.2019.120538
N. Kaur, R. Kaur, R. Kaur, S. Rana, Synthesis of novel benzothiazole based fluorescent and redox-active organic nanoparticles for their application as selective and sensitive recognition of Fe3+ ions. Inorg. Chem. Commun. (2021). https://doi.org/10.1016/j.inoche.2021.108648
R. Ferreira, J. Chaar, M. Baldan, N. Braga, Simultaneous voltammetric detection of Fe3+, Cu2+, Zn2+, Pb2+ e Cd2+ in fuel ethanol using anodic strip** voltammetry and boron-doped diamond electrodes. Fuel (2021). https://doi.org/10.1016/j.fuel.2020.120104
N. Prabavathi, A. Nilufer, V. Krishnakumar, Quantum mechanical study of the structure and spectroscopic (FT-IR, FT-Raman, 13C, 1H and UV), NBO and HOMO-LUMO analysis of 2-quinoxaline carboxylic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 92, 325–335 (2012). https://doi.org/10.1016/j.saa.2012.02.105
M. Chaabene, B. Gassoumi, P. Mignon, R. ben Chaâbane, A.R. Allouche, New zinc phthalocyanine derivatives for nitrogen dioxide sensors: a theoretical optoelectronic investigation. J. Mol. Graph. Model 88, 174–182 (2019). https://doi.org/10.1016/j.jmgm.2019.01.008
R. Soury et al., Meso-tetrakis(3,4,5-trimethoxyphenyl)porphyrin derivatives: Synthesis, spectroscopic characterizations and adsorption of NO2. Chem. Eng. J. (2019). https://doi.org/10.1016/j.cej.2019.122005
U. Warde, N. Sekar, Fluorescent benzocoumarin-π-extended styryl hybrids: solvatochromism, excess dipole moment, NLO properties and DFT study. J. Fluoresc. 28(1), 293–309 (2018). https://doi.org/10.1007/s10895-017-2192-1
S. Badakhshan, S. Ahmadzadeh, A. Mohseni-Bandpei, M. Aghasi, A. Basiri, Potentiometric sensor for iron (III) quantitative determination: experimental and computational approaches. BMC Chem. (2019). https://doi.org/10.1186/s13065-019-0648-x
B. Gassoumi, H. Ghalla, R. ben Chaabane, Host-guest complexation studies of NO3, NO2, CO2, and N2 gas with the calix[4]arene molecule. J. Mol. Model (2020). https://doi.org/10.1007/s00894-020-04416-2
M. Chaabene et al., Spectroscopic characterization and binding interaction of heavy metal onto the surface receptor of the azobenzene: DFT and experimental approach. J. Mol. Struct. (2021). https://doi.org/10.1016/j.molstruc.2021.130962
O. Dagdag et al., Rheological, electrochemical, surface, DFT and molecular dynamics simulation studies on the anticorrosive properties of new epoxy monomer compound for steel in 1 M HCl solution. RSC Adv. 9(8), 4454–4462 (2019). https://doi.org/10.1039/c8ra09446b
M. Echabaane, A. Rouis, I. Bonnamour, H. ben Ouada, Studies of aluminum (III) ion-selective optical sensor based on a chromogenic calix[4]arene derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 115, 269–274 (2013). https://doi.org/10.1016/J.SAA.2013.06.053
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. (2010). https://doi.org/10.1063/1.3382344
Y.A. Parr, B. Miehlich, A. Savin, H. Stoll, H. Preuss, Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 157, 200–206 (1989)
A.D. Becke, Density-fnnctional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988)
G. Chopra, N. Chopra, D. Kaur, Elucidating the intermolecular hydrogen bonding interaction of proline with amides—quantum chemical calculations. Struct. Chem. 30(3), 755–767 (2019). https://doi.org/10.1007/s11224-018-1235-9
Acknowledgements
Financial support of this research was received from the Ministry of Higher Education and Scientific Research of Tunisia
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moulahi, N., Echabaane, M., Chaabene, M. et al. Impedimetric sensor for iron (III) detection based on small molecule (E)-2-((phenylimino)methyl) phenol-modified platinum electrode. J IRAN CHEM SOC 20, 1427–1438 (2023). https://doi.org/10.1007/s13738-023-02767-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02767-0