Abstract
The interaction of CH2O and SO2 molecules with TiO2 anatase nanocrystals were studied using the density functional theory calculations. Several adsorption positions of CH2O and SO2 molecules on the TiO2 surface were examined in detail. The results include the calculations of the adsorption energies, electronic structures and structural parameters of the adsorption systems. We found that both oxygen and carbon atoms of the CH2O molecule interact with TiO2 surface, while the hydrogen atoms does not contribute to the adsorption process. Besides, the oxygen atom of SO2 molecule strongly interacts with the TiO2. The adsorption of CH2O and SO2 on the N-doped surface is more favorable in energy than the adsorption on the pristine surface, suggesting that the N-doped nanocrystal acts as an appropriate sensing material. The substantial changes in the electronic structure near the fermi level reveal that the nitrogen modified TiO2 would be a promising sensing material for CH2O and SO2 detection. The charge density difference calculations reveal that the electronic density increases at the middle of the newly formed bonds, as evidenced by the significant overlaps of the projected density of states between the interacting atoms. Besides, the distribution of spin densities reveals that the magnetization was mainly located on the adsorbed CH2O molecule, being useful for the design and characterization of highly efficient gas sensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a promising photocatalyst, TiO2 has attracted great attention because of its outstanding properties such as chemical stability, non-toxicity, low cost and strong oxidizing ability. TiO2 photocatalysis has been widely applied in environmental chemistry and related fields, such as wastewater treatment, sterilization, surface self-cleaning and purification of air [1,2,3,4]. The photocatalytic activity of TiO2 is very important feature, which can be modified by several strategies. Among the most important strategies, surface properties noticeably play a key role. The adsorption of different reactants and electron transfer at the interface between the solid adsorbent and the reactant are important steps in a photocatalytic process [5, 6]. Moreover, the surface stability and reactivity are assumed to be critical for the high energy conversion efficiency [7]. TiO2 exists in three naturally occurring crystallographic polymorphs: anatase, rutile and brookite. TiO2 anatase showed an improved photocatalytic activity over rutile and brookite phases [8]. There are four important facets in the TiO2 anatase specified by (001), (101), (010) and (100) facets. Of the most important facets, (101) surface is the most widely studied one in different surface processes. However, the (001) surface of TiO2 has more photocatalytic reactivity than (101) surface [9,10,11].
Lu et al. [23] suggested that the (001) surface of TiO2 anatase has higher photocatalytic activity than (101) surface via a simple photoelectrochemical method. Several researches have been also devoted to the interaction of the (001) surface of TiO2 with some molecules such as water, oxygen or other small molecules, demonstrating high reactivity of TiO2(001) surface. For instance, Gong et al. [12, 13] showed that the dissociative adsorption of formic acid on the (001) facet of TiO2 anatase is more energetically favorable than the adsorption on the other facets. It is expected that the improved reactivity of TiO2 anatase nanoparticles mainly originates from its (001) surface. Recently, Li et al. [14] discovered that the platinum nanoparticles decorated (001) surface of TiO2 could significantly improve the photocatalytic activity for degradation of CH2O molecules. Especially, Hummatov et al. [15] revealed the improved adsorption of NO x and SO x molecules on the anatase supported BaO and Pt overlayers. Furthermore, Schneider et al. [16] performed a combined computational and experimental study on the adsorption of SO x molecules on the MgO nanoparticles. More recently, Tang et al. [17, 18] studied the adsorption of NO x molecules on Pt decorated graphene oxides. Beyond that, TiO2-based composites have been demonstrated to be highly sensitive gas sensors for the removal of harmful air pollutants from the atmosphere [19,20,21,22,23,24,25,26,27,28,29].
Gas sensing with semiconducting metal oxide nanostructures has aroused considerable attentions in the past few years. In this regard, TiO2-based nanostructures were characterized as efficient candidates for versatile and inexpensive gas sensors [30]. The gas sensing capability of TiO2 has been investigated in detail, and the effect of ultraviolet (UV) irradiation on the resistance response has been examined [31, 32]. Recognition of hazardous and toxic compounds such as CH2O and SO2 molecules by metal oxide based sensors is an important issue, which significantly affects the environmental protection and health.
Formaldehyde (CH2O) molecule causes detrimental effects on the human body due to its toxicity and volatility. CH2O mainly originates from the interior building materials, synthetic panels, furniture, paint and textiles. It is also one of the most important indoor air pollutants. CH2O makes severe respiratory tract irritation, edema and headache, and even worse to make death [33]. Several methods for the removal of harmful CH2O molecule from indoor environment have been proposed including adsorption on the different surfaces, photocatalytic oxidation, plasma technology and thermal catalytic oxidation [34].
In the recent years, substantial techniques have been examined for the design and characterization of catalysts that selectively reduce the concentration of CH2O and SO2 molecules in the environment. However, TiO2 as a highly efficient sensing material plays a significant role and holds great potential for the development of sensitivity to reduce the harmful gases in the atmosphere. In this work, we presented theoretical insights into the effects of nitrogen do** on the adsorption behaviors of TiO2-based nanostructures for the detection of CH2O and SO2 molecules. In this regard, we performed several important analyses including density of states, molecular orbitals, charge density differences and distribution of spin densities. Moreover, the charge transfer between adsorbate and TiO2 nanocrystal was evaluated based on the Mulliken population analysis. Our theoretical insights shed light on the potential capability of N-doped TiO2 nanocrystals as highly sensitive gas sensors.
Methods and models
Methods
The electronic structures and energetics of the stoichiometric TiO2 anatase nanocrystals were examined using the density functional theory (DFT) [35, 36] calculations, as implemented in the open-source package for material eXplorer (OPENMX3.8) [37]. The pseudo-atomic orbitals (PAOs) centered on atomic sites were used as basis functions [38,39,40]. The energy cutoff was set to 150 Ry to achieve the precision of 10−6 Ry/atom. Basis functions were made using a confinement scheme and chosen as the followings: Ti7.0-s3p3d1, O5.0-s2p2, N5.0-s2p2, C5.0-s2p1 and H5.0-s2. Ti, O, N, C and H specify the atomic symbols of the considered elements, and the values related to the atomic symbols represent the cutoff radius in the units of the Bohr radius. The last set of symbols defines the employment of different primitive orbitals for building basis sets. The generalized gradient approximation (GGA) with the functional of Perdew–Burke–Ernzerhof (PBE) was used to treat the exchange–correlation interaction [41].
Since the binding is expected to be important for the systems considered here, we have implemented all of the calculations using the Grimme’s DFT-D2 method. This method has been developed to take the effects of long range van der Waals (vdW) interactions into account, indicating the energetics and most stable geometries of the adsorbates over the substrate [42]. The criterion for self-consistent electronic minimization was set to 10−6 Hartree. The open-source program, XCrysDen, Koklj [43], was used for visualization of the adsorption configurations. Moreover, atomic and electronic structure analyses were performed using the VESTA code (visualization for electronic and structural analysis), which presents a better visualizing of the volumetric data such as electron/nuclear densities and HOMO–LUMO diagrams [44]. CH2O molecule possesses a trigonal planar with C–O and C–H bond lengths of 1.21 and 1.08 Å, respectively. All of these calculated values are in reasonable agreement with the previous theoretical results [45].
The term desorption energy (∆Ead) was used to describe the binding of the gas molecule to the TiO2 nanocrystal. The adsorption energies are calculated using the following equation:
Here E(NC+adsorbate) is the total energy of the nanocrystal with adsorbed gas molecule, ENC is the energy of a bare TiO2 nanocrystal without gas molecule, and Eadsorbate represents the energy of an isolated gas molecule. The negative value of adsorption energy represents that the reaction between adsorbent and adsorbate is more energetically favorable.
Nanocrystal models
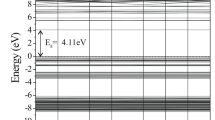
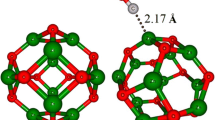
The considered TiO2 anatase nanocrystals were constructed from the bulk anatase crystal. The unit cell of TiO2 was taken from “American Mineralogists Database” webpage [46], reported by Wyckoff [47]. It is well known that various types of TiO2 nanoparticles were studied in the literature including spherical nanoparticles (nanospheres) and faceted nanoparticles (nanocrystals). Faceted nanoparticles or nanocrystals (NC) were constructed from the bulk anatase crystal based on the minimum energy shape predicted by Barnard et al. [48]. When cutting the nanocrystal, excess atoms were detached from the surface. This leads to the creation of stoichiometric nanocrystals with an appropriate number of contributing atoms. Spherical nanoparticles or nanospheres (NS) were also constructed by cutting an appropriate sphere with the considered radius (RM) from the bulk anatase crystal [49]. Since the surface energy of (101) facet of TiO2 anatase is lower than the surface energies of (100) and (001) facets, the bipyramid particles with eight {101} facets [50] were considered here. The considered anatase nanocrystal contains 105 atoms (35 Ti and 70 O atoms) of undoped or N-doped TiO2. The considered TiO2 particle possesses two dangling oxygen atoms at the edge of {001} facets. The widths of the particles in x, y, z directions are calculated to be 8.2148, 8.2148 and 21.9636 Å, respectively. As a result, the volume of the system would be 182.2793 Å3. The size of the studied TiO2 nanocrystal was chosen following the results obtained by Lei et al. [1.
Results and discussion
CH2O adsorption on TiO2 nanocrystals
We have studied the adsorption of CH2O molecule on the undoped and N-doped TiO2 anatase nanocrystals based on various adsorption sites. CH2O molecule can adsorb on the nanocrystal surface, especially on the fivefold coordinated titanium site. Figures 2 and 3 show the optimized geometry configurations of CH2O molecule on the N-doped and undoped nanocrystals, respectively. In configurations A and C, it can be seen that the carbon and oxygen atoms of the CH2O molecule coordinated to the doped nitrogen and fivefold coordinated titanium atoms, providing a double contacting point. Configuration B presents a single contacting point, indicating the coordination of the oxygen atom of CH2O to the titanium atom of TiO2. For CH2O adsorption on the pristine nanocrystal, there is only one interacting point, and the oxygen atom of CH2O molecule was bonded to the titanium atom. The results of the bond lengths and angles for CH2O adsorption on the TiO2 nanocrystals are summarized in Table 1. It can be seen from this table that the C–H and C–O bonds of the adsorbed CH2O molecule were elongated after the adsorption process. It suggests that –H and C–O bonds were weakened upon CH2O adsorption. The adsorption energies for CH2O adsorption on the considered nanocrystals are also listed in Table 1. As can be seen from this table, the N-doped nanocrystals have higher adsorption energies than the undoped ones, representing that CH2O strongly interacts with the N-doped TiO2 nanocrystals. Besides, the adsorption on the N-doped nanocrystals is more favorable in energy than that on the pristine ones. Over the pristine particle, the adsorption process is weakly favored. In order to further describe the electronic structures of the adsorption systems, we have calculated the projected density of states (PDOS) for CH2O adsorbed TiO2 nanocrystals. Figure 4 presents the PDOSs for configurations A–C. As can be seen from this figure, there are substantial overlaps between the PDOS spectra of the titanium and oxygen, as well as the doped nitrogen and carbon atoms. These strong overlaps confirm the formation of strong chemical bonds between the interacting atoms. In this case, the titanium and doped nitrogen atoms of the nanocrystal form chemical bonds with the oxygen and carbon atoms of the CH2O molecule, respectively.
The molecular orbital calculations were also carried out to further analyze the electronic properties of the system. The isosurfaces of the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) are displayed in Fig. 5. Figures S1 and S2 also present the isosurface plots for other configurations. As can be seen, the electronic densities in the HOMOs were mainly distributed over the adsorbed CH2O molecule, whereas in the LUMOs, the electronic densities were mainly concentrated on the TiO2 nanocrystal. We have also calculated the spin-polarized density of states and spin density distributions for CH2O adsorbed TiO2 nanocrystals. The spin-polarized PDOS is displayed in Figure S3, and the related spin density plots are shown in Figs. 6 and 7. It can be seen from these figures that the magnetization was mainly located over the adsorbed CH2O molecule.
To further elucidate the charge transfer between CH2O molecule and TiO2 nanocrystal, we have calculated the charge (spin) density difference caused by the interaction between CH2O molecule and TiO2. The charge density difference plots for CH2O adsorbed complexes are depicted in Figs. 8 and 9. The charge difference is calculated via subtracting the electron densities of the isolated components (CH2O and TiO2) from the charge density of the combined (TiO2 + CH2O) system. We have calculated the composite system consisting of TiO2 and CH2O molecule, and non-interacting isolated systems. The coordinates of the non-interacting TiO2 and CH2O components are the same as those in the composite TiO2 + CH2O system. The term “charge density difference” is calculated using the following formula:
where ρ(composite+adsorbate), ρ (composite) and ρ (adsorbate) represent the total charge densities of the combined TiO2 + CH2O, bare TiO2 and free CH2O molecule, respectively. The charge density difference plots show that there is lower electronic density accumulation on the adsorbed CH2O molecule. On the other hand, we can see a noticeable charge depletion over the surface, suggesting its charge donor property, as evidenced by the Mulliken charge analysis. Besides, the electron density increases at the middle of the newly formed bonds. As a result, CH2O adsorption on the nanocrystal surface is a chemical adsorption because of the formation of chemical bonds. The charge transfer from the CH2O molecule to the TiO2 indicates that TiO2 nanocrystal acts as a promising sensor device for CH2O detection.
Isosurface plot of the electron charge density difference for CH2O on the considered TiO2 nanocrystal (configuration A) with the isovalue of ± 0.0002 a.u. (top view and side view are provided in order to give a better understanding of the charge transfer at the interface). The charge accumulation is denoted in cyan, and charge depletion is in yellow, respectively
SO2 adsorption on TiO2 nanocrystals
SO2 molecule reacts with the TiO2 anatase nanocrystals by forming chemical bonds. The fivefold coordinated titanium atoms were also considered as the most stable sites. Thus, the oxygen atoms of the SO2 molecule were characterized as the binding sites on the SO2 molecule, whereas on the TiO2 nanocrystal, the binding sites were mainly located on the titanium atoms. The optimized configurations of SO2 on the TiO2 anatase nanocrystals are shown in Fig. 10, as denoted by adsorption types A–F. Configurations A–D represent the adsorption of one SO2 molecule on the pristine and N-doped nanocrystals, while configurations E and F show the adsorption of two and four SO2 molecules on the surface. The variation of bond lengths and angles is listed in Table 2, which indicates that the S–O bonds of the adsorbed SO2 molecule were elongated after the adsorption process. This elongation of bond length could be mostly attributed to the transfer of electronic density from the S–O bonds and the TiO2 nanocrystal to the newly formed bonds between the nanocrystal and SO2 molecule. The adsorption energies for SO2 on the TiO2 anatase nanocrystals are summarized in Table 2. The results suggest that SO2 adsorption on the N-doped nanocrystals (configurations A–C) is more energetically favorable then that on the undoped one (configuration D). Hence, the N-doped configurations are more stable than the pristine ones for SO2 adsorption. As can be seen from Table 2, the highest adsorption energy belongs to configuration A, representing SO2 adsorption on the N-doped TiO2 nanocrystal. Generally, the adsorption energies of configurations E and F are higher than the adsorption energy of configuration D. This is because that configurations E and F represent the adsorption of two and four SO2 molecules on the nanocrystal. Figure 11 presents the calculated PDOS spectra of the interacting atoms for configurations A and B. The PDOSs for configurations C, D and E are also depicted in Figs. 12 and 13. As can be seen, there are significant overlaps between the PDOS spectra of the titanium atoms of TiO2 and oxygen atoms of SO2 molecule, representing the formation of chemical bonds between them. Also, the large PDOS overlaps between the sulfur atom of SO2 molecule and the doped nitrogen atom indicate the formation of strong N–S chemical bond between these two atoms. To fully describe the electronic properties, we have provided the HOMO and LUMO isosurface plots for SO2 adsorbed TiO2 complexes. Figures 14 and 15 show the isosurface plots of HOMO and LUMO for configurations C and D, respectively. It can be seen from these figures that the electronic density in the LUMOs is dominant over the adsorbed molecule, whereas the HOMOs were mainly localized on the TiO2 nanocrystal. We have also presented the isosurface plots for configurations E and F in Figs. 16 and 17, respectively. Interestingly, all LUMOs are high on the adsorbed SO2 molecule, and the electronic density in the HOMOs is mainly distributed on the TiO2 surface.
The charge density difference (CDD) calculations were also performed to further discover the electronic structure of the adsorption systems. The isosurface plots of CDDs are displayed in Fig. 18. As can be seen, the electronic density increases at the middle of the newly formed bonds. The large PDOS overlaps between the interacting atoms confirm the formation of chemical bonds. Mulliken charge analysis indicates that the charges were transferred from the TiO2 nanocrystal to the SO2 molecule, which are in agreement with the accumulation of electronic charges on the adsorbed SO2 molecule. This implies that SO2 behaves as a charge acceptor.
Conclusions
In this paper, the adsorption behaviors of CH2O and SO2 molecules on the pristine and N-doped TiO2 nanocrystals were systematically investigated using the DFT calculations. Various adsorption geometries of CH2O and SO2 on the TiO2 were examined in detail. The adsorption of CH2O and SO2 on the nanocrystals is an exothermic process. The C–O and C–H bonds of the CH2O molecule, as well as the S–O bonds of the SO2 were elongated after the adsorption process. Thus, the C–O and S–O bonds were weakened upon gas adsorption. For both CH2O and SO2 adsorption systems, it was found that the adsorption on the N-doped TiO2 nanocrystals is energetically more favorable than that on the pristine one, representing the higher reactivity of N-doped TiO2 with gas molecules. The substantial overlaps between the PDOS spectra of the interacting atoms indicate the formation of chemical bonds between them. For CH2O adsorption, the electronic density in the HOMOs is dominant over the adsorbed CH2O molecule. In contrast, for SO2 adsorption, the electronic density in the LUMOs is significant over the adsorbed gas molecule. The charge density difference calculations indicate the donor and acceptor characteristics for CH2O and SO2 molecules, respectively. Furthermore, the electronic density increases at the middle of the newly formed bonds, indicating chemical binding of gas molecules on the TiO2.
References
M. Addamo, V. Augugliaro, A.D. Paola, E.-G. López, V. Loddo, G. Marcì, R. Molinari, L. Palmisano, M. Schiavello, Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 catalysts. J. Phys. Chem. B 108, 3303–3310 (2004)
V.G. Deshmane, S.L. Owen, R.Y. Abrokwah, D. Kuila, Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn, and Sn) catalysts: effect of metal–support interactions on steam reforming of methanol. J. Mol. Catal. A 408, 202–213 (2015)
A.M. Asaduzzaman, P. Kriiger, Adsorption and cluster growth of vanadium on TiO2 (110) studied by density functional theory. J. Phys. Chem. C 112, 4622–4625 (2008)
C.W. Gong, J.R. Jiao, J.H. Wang, W. Shao, Structural, optical and magnetic properties of W-doped TiO2: theory and experiment. Phys. B Condes. Matter 457, 140–143 (2015)
A.L. Linsebigler, G.Q. Lu, J.T.Y. Jr., Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995)
M.A. Fox, M.T. Dulay, Heterogeneous photocatalysis. Chem. Rev. 93, 341–357 (1993)
U. Diebold, The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003)
M.H. Suhail, G. Mohan Rao, S. Mohan, DC reactive magnetron sputtering of titanium-structural and optical characterization of TiO2 films. J. Appl. Phys. 71, 1421–1427 (1992)
Y.B. **a, K. Zhu, T.C. Kaspar, Y.G. Du, B. Birmingham, K.T. Park, Z.R. Zhang, Atomic structure of the anatase TiO2 (001) surface. J. Phys. Chem. Lett. 4, 2958–2963 (2013)
M. Lazzeri, A. Selloni, Stress-driven reconstruction of an oxide surface: the anatase TiO2 (001)-(1 × 4) surface. Phys. Rev. Lett. 87, 266105–1–266105-4 (2001)
Y. Lu, Y.P. Zang, H.M. Zhang, Meaningful comparison of photocatalytic properties of 001 and 101 faceted anatase TiO2 nanocrystals. Sci. Bull. 61, 1003–1012 (2016)
X.Q. Gong, A. Selloni, Reactivity of anatase TiO2 nanoparticles: the role of the minority (001) surface. J. Phys. Chem. B 109, 19560–19562 (2005)
X.Q. Gong, A. Selloni, A. Vittadini, Density functional theory study of formic acid adsorption on anatase TiO2 (001): geometries, energetics, and effects of coverage, hydration, and reconstruction. J. Phys. Chem. B 110, 2804–2811 (2006)
Z.B. Li, X. Wang, L.C. Jia, X.B. **ng, Reduction of HCHO with OH on Pt loading anatase TiO2 (001) surface: a DFT calculation. Catal. Commun. 92, 23–26 (2017)
R. Hummatov, O. Gulseren, E. Ozensoy, D. Toffoli, H. Ustunel, First-principles investigation of NO x and SO x adsorption on anatase supported BaO and Pt overlayers. J. Phys. Chem. C 116, 6191–6199 (2012)
W.F. Schneider, J. Li, K.C. Hass, Combined computational and experimental investigation of SO x adsorption on MgO. J. Phys. Chem. B 105, 6972–6979 (2001)
S. Tang, Z. Cao, Adsorption of nitrogen oxides on graphene and graphene oxides: insights from density functional calculations. J. Chem. Phys 134, 044710 (2011)
S. Tang, J. Zhu, Structural and electronic properties of Pd decorated graphene oxides and their effects on the adsorption of nitrogen oxides: insights from density functional calculations. J. RSC Adv. 4, 23084–23096 (2014)
A. Abbasi, J.J. Sardroodi, A.R. Ebrahimzadeh, Chemisorption of CH2O on N-doped TiO2 anatase nanoparticle as modified nanostructure media: a DFT study. Surf. Sci. 654, 20–32 (2016)
A. Abbasi, J.J. Sardroodi, N-doped TiO2 anatase nanoparticles as a highly sensitive gas sensor for NO2 detection: insights from DFT computations. Environ. Sci. Nano 3, 1153–1164 (2016)
A. Abbasi, J.J. Sardroodi, Modified N-doped TiO2 anatase nanoparticle as an ideal O3 gas sensor: insights from density functional theory calculations. Comput. Theor. Chem. 1095, 15–28 (2016)
A. Abbasi, J.J. Sardroodi, A novel strategy for SO x removal by N-doped TiO2/WSe2 nanocomposite as a highly efficient molecule sensor investigated by van der Waals corrected DFT. Comput. Theor. Chem. 1114, 8–9 (2017)
A. Abbasi, J.J. Sardroodi, Prediction of a highly sensitive molecule sensor for SO x detection based on TiO2/MoS2 nanocomposites: a DFT study. J. Sulfur Chem. 38(1), 52–68 (2017)
A. Abbasi, J.J. Sardroodi, An innovative gas sensor system designed from a sensitive nanostructured ZnO for the selective detection of SO x molecules: a density functional theory study. New J. Chem. 41, 12569–12580 (2017)
A. Abbasi, J.J. Sardroodi, Theoretical study of the adsorption of NO x on TiO2/MoS2 nanocomposites: a comparison between undoped and N-doped nanocomposites. J. Nanostruct. Chem. 6, 309–327 (2016)
A. Abbasi, J.J. Sardroodi, Investigation of the adsorption of ozone molecules on TiO2/WSe2 nanocomposites by DFT computations: applications to gas sensor devices. Appl. Surf. Sci. 436, 27–41 (2018)
A. Abbasi, J.J. Sardroodi, Adsorption of toxic SO x molecules on heterostructured TiO2/ZnO nanocomposites for gas sensing applications: a DFT study. Adsorption 24, 29–41 (2018)
A. Abbasi, J.J. Sardroodi, A.R. Ebrahimzadeh, M. Yaghoobi, Theoretical study of the structural and electronic properties of novel stanene-based buckled nanotubes and their adsorption behaviors. Appl. Surf. Sci. 435, 733–742 (2018)
A. Abbasi, J.J. Sardroodi, Molecular design of O3 and NO2 sensor devices based on a novel heterostructured N-doped TiO2/ZnO nanocomposite: a van der Waals corrected DFT study. J Nanostruct. Chem. 7, 345–358 (2017)
N. Yamazoe, G. Sakai, K. Shimanoe, Oxide semiconductor gas sensors. Catal. Surv. Asia 7, 63 (2003)
Z. Topalian, J.M. Smulko, G.A. Niklasson, C.G. Granqvist, Resistance noise in TiO2-based thin film gas sensors under ultraviolet irradiation. J. Phys. Conf. Ser. 76, 012056 (2007)
T.Y. Yang, H.M. Lin, B.Y. Wei, C.Y. Wu, C.K. Lin, UV enhancement of the gas sensing properties of nano-TiO2. Rev. Adv. Mater. Sci. 4, 48 (2003)
J. Li, Q. Zhang, A.C.K. Lai, L.P. Zeng, Study on photocatalytic performance of cerium–graphene oxide–titanium dioxide composite film for formaldehyde removal. Phys. Status Solidi A 12, 3157–3164 (2016)
L.H. Nie, J.G. Yu, M. Jaroniec, F.F. Tao, Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 6, 3649–3669 (2016)
P. Hohenberg, W. Kohn, Inhomogeneous electron gas. Phys. Rev. 136, B864 (1964)
W. Kohn, L. Sham, Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133 (1965)
The code, OPENMX, pseudoatomic basis functions, and pseudopotentials are available on a web site http://www.openmxsquare.org. Accessed 28 May 2004
T. Ozaki, Variationally optimized atomic orbitals for large-scale electronic structures. Phys. Rev. B 67, 155108 (2003)
T. Ozaki, H. Kino, Numerical atomic basis orbitals from H to Kr. Phys. Rev. B 69, 195113 (2004)
T. Ozaki, H. Kino, Variationally optimized basis orbitals for biological molecules. J. Chem. Phys. 121, 10879 (2004)
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396 (1981)
S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27(15), 1787–1799 (2006)
A. Koklj, Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput. Mater. Sci. 28, 155–168 (2003)
K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011)
H. Liu, M. Zhao, Y. Lei, C. Pan, W. **ao, Formaldehyde on TiO2 anatase (1 0 1): a DFT study. Comput. Mater. Sci. 15, 389–395 (2012)
Web page at http://rruff.geo.arizona.edu/AMS/amcsd.php. Accessed 3 Dec 2004
R.W.G. Wyckoff, Crystal Structures, 2nd edn. (Interscience Publishers, New York, 1963)
A.S. Barnard, P. Zapol, Effects of particle morphology and surface hydrogenation on the phase stability of TiO2. Phys. Rev. B. 70, 235403 (2004)
G. Fazio, L. Ferrighi, C.D. Valentin, Spherical versus faceted anatase TiO2 nanoparticles: a model study of structural and electronic properties. Phys. Chem. C 119(35), 20735–20746 (2015)
A.S. Barnard, S. Erdin, Y. Lin, P. Zapol, J.W. Halley, Modeling the structure and electronic properties of TiO2 nanoparticles. Phys. Rev. B 73, 205405 (2006)
Y. Lei, H. Liu, W. **ao, First principles study of the size effect of TiO2 anatase nanoparticles in dye-sensitized solar cell. Model. Simul. Mater. Sci. Eng. 18, 025004-1–025004-9 (2010)
J. Liu, L. Dong, W. Guo, T. Liang, W. Lai, CO adsorption and oxidation on N-doped TiO2 nanoparticles. J. Phys. Chem. C 117, 13037–13044 (2013)
Acknowledgements
This work has been supported by Azarbaijan Shahid Madani University (Grant No: 96/235).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abbasi, A., Sardroodi, J.J. Structural and electronic properties of nitrogen-doped TiO2 nanocrystals and their effects on the adsorption of CH2O and SO2 molecules investigated by DFT. J IRAN CHEM SOC 15, 1431–1448 (2018). https://doi.org/10.1007/s13738-018-1343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1343-x