Abstract
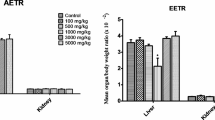
The current study evaluated the prospective toxicity of hydroalcoholic extract of Piper longum L. (HEPL) dried fruits with acute and sub-acute oral administration in Wistar rats. During acute toxicity study, female rats were orally administered with HEPL at a single dose of 300 mg/kg and repeated dose of 2000 mg/kg (OECD guidelines-423). Subacute toxicity of HEPL (250, 500, and 1000 mg/kg p.o.) was studied with the control group (1% CMC) by daily dosing in Wistar rats of both sexes for 28 days. To assess reversibility, other satellite groups were kept for another two weeks post-treatment. The acute toxicity study showed no lethal effects, and toxicity signs and LD50 transpire more significantly than 2000 mg/kg. In subacute toxicity study, oral administration of extract for 28 days; caused no significant changes in body weights; food and water consumption of rats were observed. In addition, no toxic effects of the extract on rats for hematological and biochemical parameters were observed. Histopathological analysis revealed no signs of degeneration for kidneys, testis, and ovaries; however liver showed mild multifocal hepatocellular necrosis with infiltration of inflammatory cells in rats treated with 1000 mg/kg of HEPL. These results showed that 250 mg/kg and 500 mg/kg were found safe, in addition to some liver toxicity was found for 1000 mg/kg. It is concluded that the oral LD50 of HEPL was more significant than 2000 mg/kg and is considered safe. Hence, Piper longum is safe in the short and long term for an oral dose.




Similar content being viewed by others
References
A Comprehensive Guide to Toxicology in Nonclinical Drug Development (2017) In A Comprehensive Guide to Toxicology in Nonclinical Drug Development. https://doi.org/10.1016/c2015-0-00147-2
Bajad S, Bedi KL, Singla AK, Johri RK (2001) Piperine inhibits gastric emptying and gastrointestinal transit in rats and mice. Planta Med 67(2):176–179. https://doi.org/10.1055/s-2001-11505
Bang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, Yeon JY, Yang HI, Chul MC, Hahm DH, Kim KS (2009) Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Therapy. https://doi.org/10.1186/ar2662
Benzie IFF, Wachtel-Galor S (2011) Herbal medicine: biomolecular and clinical aspects: In: Herbal medicine: biomolecular and clinical aspects, 2nd edn
Borawska MH, Markiewicz-Zukowska R, Sawicka D, Naliwajko SK, Socha K, Omeljaniuk W, Car H (2014) Effects of chrysin on haematological parameters in rats. Farmacia 62(2):390–399
Bui TT, Piao CH, Song CH, Shin HS, Shon DH, Chai OH (2017) Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell Immunol 322:64–73. https://doi.org/10.1016/j.cellimm.2017.10.005
Cajuday LA, Pocsidio GL (2010) Effects of Moringa oleifera Lam. (Moringaceae) on the reproduction of male mice (Mus musculus). J Med Plants Res 4(12):1115–1121. https://doi.org/10.5897/JMPR09.200
Dasgupta A, Datta PC (1980) Medicinal species of piper, pharmacognostic delimitation. Pharm Biol 18(1):17–25. https://doi.org/10.3109/13880208009065171
Ekor M (2014) The growing use of herbal medicines Issues relating to adverse reactions and challenges in monitoring safety. Front Neurol. https://doi.org/10.3389/fphar.2013.00177
El Hilaly J, Israili ZH, Lyoussi B (2004) Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol 91(1):43–50. https://doi.org/10.1016/j.jep.2003.11.009
Ezeja MI, Anaga AO, Asuzu IU (2014) Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J Ethnopharmacol 151(3):1155–1164. https://doi.org/10.1016/j.jep.2013.12.034
Haschek WM, Rousseaux CG, Wallig MA, Bolon B, Ochoa R (2013) Haschek and Rousseaux’s Handbook of Toxicologic Pathology. In: Haschek and Rousseaux’s handbook of toxicologic pathology. https://doi.org/10.1016/C2010-1-67850-9
Jaijoy K, Vannasiri S, Piyabhan P, Lerdvuthisopon N, Boonraeng S, Khonsung P, Lertprasertsuke N, Sireeratawong S (2010) Acute and subchronic toxicity study of the water extract from the fruits of Piper chaba hunter in rats. Int J Appl Res Nat Prod 3(4):29–35
Johri RK, Thusu N, Khajuria A, Zutshi U (1992) Piperine-mediated changes in the permeability of rat intestinal epithelial cells. The status of γ-glutamyl transpeptidase activity, uptake of amino acids and lipid peroxidation. Biochem Pharmacol 43(7):1401–1407. https://doi.org/10.1016/0006-2952(92)90195-O
Kapoor LD (2017) Handbook of ayurvedic medicinal plants: herbal reference library. In: Handbook of ayurvedic medicinal plants: herbal reference library. https://doi.org/10.1201/9780203719473
Khandelwal KR (2005) Practical pharmacognosy techniques and experiments. In: NiraliPrakashan, Pune, India (20th ed.). Nirali Prakashan Pune. Retrieved from http://www.bookganga.com/R/5FYVG
Kim HY, Zuo G, Lee SK, Lim SS (2020) Acute and subchronic toxicity study of nonpolar extract of licorice roots in mice. Food Sci Nutr 8(5):2242–2250. https://doi.org/10.1002/fsn3.1465
King GS (2002) Handbook of toxicologic pathology. In: Archives of Pathology & laboratory medicine, vol 126, No 9, pp 1138–1139. https://doi.org/10.5858/2002-126-1138b-hotp
Koduru S, Grierson DS, Afolayan AJ (2006) Antimicrobial activity of Solanum aculeastrum. Pharm Biol 44(4):283–286. https://doi.org/10.1080/13880200600714145
Koul IB, Kapil A (1993) Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med 59(5):413–417. https://doi.org/10.1055/s-2006-959721
Kunle, (2012) Standardization of herbal medicines - a review. Int J Biodivers Conserv. https://doi.org/10.5897/ijbc11.163
Lee SE, Park BS, Kim MK, Choi WS, Kim HT, Cho KY, Lee SG, Lee HS (2001) Fungicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum L., against phytopathogenic fungi. Crop Prot 20(6):523–528. https://doi.org/10.1016/S0261-2194(00)00172-1
Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y (2007) Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci 80(15):1373–1381. https://doi.org/10.1016/j.lfs.2006.12.027
Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ, Rao CA (2013) Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-13-37
OECD (2008) OECD guidelines for the testing of chemicals: repeated dose 28-day oral toxicity study in rodents. Drug Chem Toxicol 34(1):13. Retrieved from http://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en
Patil UH, Gaikwad DK (2010) Phytochemical profile and antibacterial activity of stem bark of Anogeissus latifolia. Pharmacognosy J 2(17):70–73. https://doi.org/10.1016/S0975-3575(10)80014-8
Pieme CA, Penlap VN, Nkegoum B, Taziebou CL, Tekwu EM, Etoa FX, Ngongang J (2006) Evaluation of acute and subacute toxicities of aqueous ethanolic extract of leaves of Senna alata (L.) Roxb (Ceasalpiniaceae). Afr J Biotechnol 5(3):283–289. https://doi.org/10.5897/AJB05.197
Piyachaturawat P, Glinsukon T, Toskulkao C (1983) Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol Lett 16(3–4):351–359. https://doi.org/10.1016/0378-4274(83)90198-4
Porwal M, Khan NA, Maheshwari KK (2017) Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci Pharm. https://doi.org/10.3390/scipharm85030029
Raina P, Chandrasekaran CV, Deepak M, Agarwal A, Ruchika KG (2015) Evaluation of subacute toxicity of methanolic/aqueous preparation of aerial parts of O. sanctum in Wistar rats: clinical, haematological, biochemical and histopathological studies. J Ethnopharmacol 175:509–517. https://doi.org/10.1016/j.jep.2015.10.015
Ricchiuti V (1999) Tietz textbook of clinical chemistry. Am J Clin Pathol 112(1):120–121. https://doi.org/10.1093/ajcp/112.1.120
Rogić D (2008) Tietz fundamentals of clinical chemistry. In: Biochemia medica: Biochemia medica, vol 18, No 3
Shenoy PA, Nipate SS, Sonpetkar JM, Salvi NC, Waghmare AB, Chaudhari PD (2013) Anti-snake venom activities of ethanolic extract of fruits of Piper longum L. (Piperaceae) against Russell’s viper venom: characterization of piperine as active principle. J Ethnopharmacol 147(2):373–382. https://doi.org/10.1016/j.jep.2013.03.022
Shoji N, Umeyama A, Saito N, Takemoto T, Kajiwara A, Ohizumi Y (1986) Dehydropipernonaline, an amide possessing coronary vasodilating activity, isolated from Piper iongum L. J Pharm Sci 75(12):1188–1189. https://doi.org/10.1002/jps.2600751215
Stöhr JR, **ao PG, Bauer R (2001) Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro. J Ethnopharmacol 75(2–3):133–139. https://doi.org/10.1016/S0378-8741(00)00397-4
Ugwah-Oguejiofor CJ, Okoli CO, Ugwah MO, Umaru ML, Ogbulie CS, Mshelia HE, Umar M, Njan AA (2019) Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01179
Vasudevan K, Vembar S, Veeraraghavan K, Haranath PSRK (2000) Influence of intragastric perfusion of aqueous spice extracts on acid secretion in anesthetized albino rats. Indian J Gastroenterol 19(2):53–56
Vedhanayaki G, Shastri GV, Kuruvilla A (2003) Analgesic activity of Piper longum Linn. root. Indian J Exp Biol 41(6):649–651
Zaveri M, Khandhar A, Patel S, Patel A (2010) Chemistry and pharmacology of Piper longum L. Int J Pharm Sci Rev Res 5(1):67–76
Acknowledgements
Authors are grateful to Maratha Vidya Prasarak Samaj’s College of Pharmacy, MVP Campus, Gangapur road, Shivaji Nagar, Nashik and Crystal Biological Solutions, Pune for providing all the facilities to complete this research work.
Funding
The authors declare that this study has no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The study was approved by the Institutional Animal Ethics Committee (Protocol No: MVPCP/IAEC/2019/05) of Maratha Vidya Prasarak Samajs College of Pharmacy, Nashik constituted under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India. According to established general guidelines in Guide for Care and Use of Laboratory Animals, ethical guidelines were strictly followed.
Conflict of interest
Swati Vinod Jogdand has no conflict of interest. Ghanshyam B. Jadhav has no conflict of interest. Yogesh P. Talekar has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jogdand, S.V., Jadhav, G.B. & Talekar, Y.P. Acute and sub-acute toxicity studies of hydro-alcoholic extract of dried fruits of Piper longum Linn in Wistar rats. ADV TRADIT MED (ADTM) 24, 179–190 (2024). https://doi.org/10.1007/s13596-023-00680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-023-00680-8