Abstract
Key message
Multiple lines of evidence suggest acoustic wave velocity (AWV) would provide a rapid and efficient method to indirectly select for superior pulp yield in Eucalyptus globulus breeding programs.
Context
Eucalyptus globulus is one of the most widely planted hardwood species in temperate regions of the world and is primarily grown for pulpwood.
Aims
To determine if acoustic wave velocity (AWV) can be used to indirectly select for kraft pulp yield in E. globulus.
Methods
Genetic group effects, additive and non-additive variance components, and genetic correlations were estimated for AWV and pulpwood traits, including Kraft pulp yield. In a separate trial, the relative position of quantitative trait loci (QTL) for these traits was compared.
Results
Estimated narrow-sense heritabilities for AWV and pulp yield were both 0.26, and these traits were strongly genetically correlated (0.84). Furthermore, co-located QTL for these traits were identified. Further evidence that AWV could be used to indirectly select for pulp yield was provided by the ranking of genetic groups—Otways and King Island had the highest AWV and pulp yield and Strzelecki and Tasmania the lowest. There was no evidence of dominance variation in wood property traits.
Conclusion
Together, these findings suggest that AWV could be used as a selection criterion for kraft pulp yield in E. globulus breeding programs.
Similar content being viewed by others
1 Introduction
Eucalyptus globulus is one of the most widely planted hardwood species in temperate regions of the world, with extensive commercial plantations in Australia, Chile, Uruguay, Spain and Portugal. The species has excellent kraft pul** properties and is primarily grown for this purpose. However, there is an increasing interest in growing the species for solid-wood products, including sawn timber and engineered wood products (Hamilton et al. 2015b; Potts et al. 2013). Growth rate and pulp yield are economically important tree characteristics for vertically integrated kraft pulpwood growers and, along with basic density, are key targets for improvement in E. globulus breeding programs (Potts et al. 2013). These traits are usually assessed in breeding populations at between 4 and 8 years of age using a variety of tools including near infrared reflectance spectroscopy (NIR), which is a non-destructive means of estimating wood chemical properties (Downes et al. 2009).
Positive genetic correlations among acoustic wave velocity (AWV), pulp yield and basic density have been identified in E. nitens (Blackburn et al. 2012), a species closely related to E. globulus, suggesting that AWV may represent a cheaper alternative to NIR or be used as a means of identifying candidates for NIR in that species (Blackburn et al. 2014; Raymond et al. 2010). Acoustic velocity assessment can be undertaken directly using a microsecond timing device or with a resonance tool (refer to Raymond et al. 2010). Traditional interest in the assessment of AWV has been as an indirect measure of wood stiffness as it is positively correlated with modulus of elasticity (MOE), a key structural characteristic indicative of end-product stiffness (Blackburn et al. 2012; Blackburn et al. 2010). Furthermore, favourable phenotypic correlations among AWV, MOE and pulp yield in eucalypts (Dickson et al. 2003; Downes et al. 2008; Raymond et al. 2010) suggest acoustic assessments could be used to segregate logs into stiffness and/or pulp yield classes at mills, log landings or at harvest, by incorporating sensors into processing heads (Amishev et al. 2010). Such segregation would allow logs to be allocated to their most profitable use, can reduce the cost of handling, transporting and processing unsuitable logs and/or can improve the efficiency of processing (Raymond et al. 2010).
The primary objective of this study was to examine the potential to use standing tree AWV to indirectly select for kraft pulp yield in E. globulus. We used outcrossed full-sib families and quantitative genetic approaches to quantify the additive and non-additive genetic variation in, and estimate genetic correlations among, these traits as well as diameter and cellulose content. To further characterise the genetic relationships between AWV and these traits, quantitative trait loci (QTL) for acoustic wave velocity were also detected and compared with previously reported QTL for growth and wood properties in the same map** families.
2 Materials and methods
2.1 Genetic material and trial designs
Two adjoining unthinned and unpruned E. globulus genetic trials, herein referred to as Trial 1 and Trial 2, were studied. These trials were the subject of a previous study of growth and harvesting traits (Hamilton et al. 2015a). Data from Trial 1 were used to estimate quantitative genetic parameters, and data from Trial 2 were used for QTL analyses. These trials were established on an ex-pasture site with a slight west-north-westerly aspect (~4.5°) by the Southern Tree Breeding Association (STBA) on a Western Australian Plantation Resources (WAPRES) property near Manjimup, Western Australia (34° 14′ 52″ S, 116° 3′ 32″ E) in 1991. Manjimup experiences a Mediterranean climate with an average annual rainfall of 1007 mm.
Trial 1 was comprised of 11 replicates, each with six 4 × 6 tree blocks of ‘cross-type’ treatments with different levels of inbreeding: self-pollinated (one block per replicate), open-pollinated seed-orchard (one block per replicate) and full-sib out-crossed families (four blocks per replicate). Within replicates, cross-type treatments were randomly allocated to blocks and, within blocks, families were planted as single-tree plots. In the case of full-sib out-crossed families, an incomplete-block trial design was imposed (i.e. ‘blocks’ were treated as ‘incomplete-blocks’ within replicates). To estimate genetic parameters unbiased by variable levels of inbreeding and inbreeding depression (Costa e Silva et al. 2010), only data from full-sib out-crossed families were used in the current study. Insufficient seedlings of some out-crossed families were available at the time of planting and their plantation positions were filled with families with excess individuals.
In total, 166 full-sib out-crossed families from 178 parents were represented in Trial 1. The parents were from 12 subraces (Dutkowski and Potts 1999) which were consolidated into five genetic groups due to the low genetic contribution of some subraces (Table 1). Survival at the time of assessment was 91%.
Trial 2 comprised nine full-sib out-crossed F1 families, three of which were used for QTL analysis. From each family, a total of 92 individuals from 4 to 5 replicate 5 × 5 plots were used for QTL analysis, which were the same individuals as used in an earlier QTL study (Freeman et al. 2013). In combination, these families sampled a diverse section of the natural distribution of E. globulus. The trial was designed according to a modified row-column design at the plot level, where over-represented families were randomly assigned to plot positions of under-represented families. (see Freeman et al. 2013 and Hamilton et al. 2015a, for more detailed descriptions of pedigrees and trail designs).
2.2 Traits assessed
At Trial 1, standing tree AWV was assessed with a single-pass Fakopp Microsecond Timer prior to harvest 10 years after planting. Fakopp probes were placed at 0.5 and 1.7 m, one directly above the other, on the eastern side of each stem. Probes were positioned to minimise the number of branches and/or branch stubs between them and driven into the wood to a depth of approximately 12 mm below the bark—the upper probe pointing down and the lower probe pointing up at approximately 45° to the stem. The Fakopp Microsecond Timer measures the time taken for an acoustic stress wave to pass from one probe to the other, allowing estimation of AWV based on the distance between the probes. Outer-wood breast height drill swarf samples (approximately 40 mm depth; Meder et al. 2010) were then taken for NIR prediction of Kraft pulp yield (Kappa 18) and cellulose content (Downes et al. 2011). Swarf samples were air-dried and ground in a Wiley mill to provide a 16-mesh woodmeal. This gave a sample sufficiently fine enough to ensure the portion from which spectra were collected was representative of the whole sample. Ground samples were stored and analysed at ambient laboratory conditions. Woodmeal from each of these samples were used to obtain NIR spectra between 4000 and 10,000 wave-numbers (1000–2500 nm) on a Bruker MPA FT-NIR instrument. Predictions of pulp yield and cellulose content were then made using previously published calibrations (Downes et al. 2011). Stem diameter over bark of the main stem of each tree was measured at breast height (1.3 m; DBH).
At Trial 2, standing tree AWV was assessed at the same time and using the same procedure as Trial 1. These data were used in combination with historic wood chemistry, wood density and growth data collected at age 7 years for genetic map** and QTL analysis (refer to Freeman et al. 2013).
2.3 Estimation of quantitative genetic parameters
To estimate variance components, univariate restricted maximum likelihood (REML) analyses were undertaken separately for each trait using data from out-crossed families in Trial 1 and the following linear mixed model:
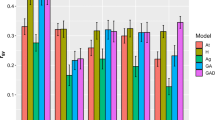
where y is the vector of trait observations, b is the vector of fixed effects with its design matrix X, u is the vector of random effects with its design matrix Z and e is the vector of random residual terms. The models included as fixed effects in b the overall mean and replicate. The random effects in u were incomplete block within replicate, genetic group general combining ability (GCA), genetic group specific combining ability (SCA), the additive genetic component within genetic group and full-sib family within genetic group (refer to Hamilton et al. 2015a). For each trait, the narrow-sense heritability (h2), coefficient of additive genetic variance (CVa), dominance variance \( {\upsigma}_{\mathrm{d}}^2 \) and dominance ratio (d2) were estimated from univariate analyses as follows:
where \( \overset{-}{x} \) is the trait mean, \( {\upsigma}_{\mathrm{a}}^2 \) is the additive genetic variance, \( {\upsigma}_{\mathrm{f}}^2 \) is the non-additive full-sib family-specific variance and \( {\upsigma}_{\mathrm{e}}^2 \) is the residual variance. A bivariate model was used to estimate inter-trait genetic correlations (refer to Hamilton et al. 2015a). Variances for random effects that were not significantly different from zero at the P = 0.10 level in univariate analyses were fixed to zero in bivarate analyses. Two-tailed likelihood ratio tests were used to test if genetic correlations were significantly different from zero and one-tailed likelihood ratio tests were used to determine if these correlations were significantly different from one (Gilmour et al. 2009). Standard errors of parameters were estimated from the average information matrix, using a standard truncated Taylor series approximation (Gilmour et al. 2009).
Pearson correlation-coefficients among individual-tree phenotypic values, herein referred to as phenotypic correlations, were also estimated. Two-tailed t tests were used to test if phenotypic correlations were significantly different from zero. Analyses were conducted using ASReml™ Version 3.0 (Gilmour et al. 2009) and SAS™ (version 9.1).
2.4 Analysis of quantitative trait loci
This study expanded on previous QTL analyses for growth and wood properties in the same F1 families (Freeman et al. 2013). Analyses of AWV QTL were conducted with MapQTL 6.0 (Van Ooijen and Kyazma 2009), using the consensus linkage map described in Freeman et al. (2013). MapQTL 6.0 (Van Ooijen and Kyazma 2009) default parameters were used for all analyses. Putative QTL were declared at a logarithm of odds (LOD) threshold of 3, which equated to an average chromosome-wide Type I error rate of <0.05, determined by permutation testing in each family (1000 replications; Churchill and Doerge 1994). It should be noted, this threshold is more conservative than the threshold for ‘suggestive QTL’ (i.e. chromosome-wide Type I error rate of <0.1) adopted by Freeman et al. (2013). Interval and multiple-QTL model (MQM) map** were performed as described by Freeman et al. (2013) but used the regression algorithm now available in MapQTL 6.0 (Van Ooijen and Kyazma, 2009).
To compare the genetic architecture underlying variation in AWV, growth and wood properties, the QTL for AWV were positioned on the map presented by Freeman et al. (2013) (Table 2, Fig. 1). This included QTL for chemical wood properties (kraft pulp yield, cellulose, Klason lignin, lignin S:G ratio and extractives content; each predicted using near-infrared spectroscopy), basic density and diameter at breast height, measured at 7 years of age. To aid the comparison of QTL from the present study with those previously detected, QTL were mapped using 15 cM intervals, following Freeman et al. (2013). Co-located QTL are defined as those with overlap** 15 cM intervals (Fig. 1). In addition to Trial 2 at Manjimup, Freeman et al. (2013) also included trees from the same F1 families planted at Branxholme in western Victoria (n = 183–184 trees per family, half at each trial site) and conducted analyses within each F1 pedigree site by site, and combined across sites (Table 2).
Quantitative trait loci (QTL) for acoustic wave velocity, as well as QTL previously identified for wood properties and growth, in three F1 families of Eucalyptus globulus. Each QTL is represented by an arbitrary 15 cM bar surrounding the QTL peak, where QTL maps within 7.5 cM of the end of a linkage group the end is truncated. ‘w’ and ‘v’ indicate QTL significant only in the Western Australian and Victorian trial sites, respectively. Candidate gene positions are indicated to the left of each LG, see Freeman et al. (2013) for more details
3 Results
In the quantitative genetic analyses (Trial 1), a significant (P < 0.05) genetic group GCA effect was observed in AWV, pulp yield and cellulose content (Table 3). The genetic group SCA effect was significant in the case of AWV only. King Island exhibited the highest AWV, and Tasmania the lowest (Table 4). For pulp yield and cellulose content, Otways and King Island ranked highest, and Strzelecki lowest. The within genetic group additive genetic variance was significantly different from zero for all traits but the dominance variance was significant for diameter only (Table 2; Hamilton et al. 2015a). Narrow-sense heritabilities ranged from 0.15 for diameter to 0.26 for both AWV and pulp yield and coefficients of additive genetic variation ranged from 1.9% for pulp yield to 12.6% for diameter.
Both phenotypic and additive genetic correlations among traits were positive and significantly different from zero in all cases except for the additive genetic correlation between pulp yield and diameter (Table 5). At the phenotypic level, diameter was significantly positively correlated with AWV, pulp yield and cellulose content (0.55–0.63). The additive genetic correlations among wood properties were very strong (≥0.84), including that between pulp yield and AWV. There was no evidence to indicate that the strength of this correlation was an artefact of a common relationship with diameter as: (i) the genetic correlation between pulp yield and diameter, although moderate (0.38), was not significantly different from zero; and (ii) when the analysis used to estimate the genetic correlation between AWV and pulp yield was modified to include diameter as a covariate, the additive genetic correlation remained strong and significantly different from zero (0.80; P < 0.001).
In the QTL analysis (Trial 2), six discrete QTL were identified for AWV in total, three in each of the Families 4 and 5 (Table 2 and Fig. 1). No significant QTL were detected in Family 1. The QTL were distributed across four linkage groups (LG) and included those of moderate to high significance (P < 0.05–P < 0.001) on linkage groups 3, 5 and 10. Three of the six QTL for AWV co-located with those for pulp yield (Fig. 1—Family 4 LG5 and Family 5 LG3 and LG10), two of which involved co-location within the same family (Family 4 LG5 and Family 5 LG10). In both cases, the co-located QTL for AWV segregated from the same parent as the QTL for pulp yield, and one of these (Family 4 LG5) was independent of QTL for other wood properties. Two of the six AWV QTL (Family 4 LG3 and Family 5 LG10) co-located with wood property candidate genes (Table 2). While AWV QTL also co-located with other wood property traits, such as density and Klason lignin, the overall highest consistent Pearson phenotypic correlation observed within each family was with pulp yield (Table 6).
4 Discussion
Two lines of evidence indicated a genetic relationship between acoustic wave velocity (AWV) and NIR-predicted kraft pulp yield in E. globulus. Firstly, quantitative genetic analyses revealed that AWV and pulp yield were both under additive genetic control, and the additive genetic correlation between these traits was strongly positive (0.84). This correlation estimate appeared to be robust, because it was based on control pollinated seedlots, and its standard error was small. Furthermore, a similarly strong and positive additive genetic correlation between AWV and pulp yield was found in E. nitens (0.73; Blackburn et al. 2012). However, estimates of genetic correlations are notoriously variable across studies (Hamilton and Potts 2008), and further investigation is required to determine if this relationship is consistent across populations, environments, ages and different methods of measurement in E. globulus and other pulpwood species. Nevertheless, a positive genetic relationship between AWV and pulp yield was also reflected in the ranking of genetic groups—Otways and King Island had the highest AWV and pulp yield, and Strzelecki and Tasmania the lowest.
Secondly, AWV and pulp yield QTL were co-located in two independent families. Co-located QTL may reflect the influence of pleiotropic regulators influencing many developmental and/or biosynthetic pathways (Kirst et al. 2005) or linked clusters of genes (Breitling et al. 2008). However, segregation from the same parent in each case was consistent with pleiotropy. An additional QTL, on LG 3, was exactly co-located with a cellulose synthase gene CSA3 (Fig. 1), which has been linked to variation in density in E. globulus in a previous study (Thavamanikumar et al. 2014), could reflect a pleiotropic influence of this locus upon cellulose/pulp yield and AWV.
Taken together, our QTL and quantitative genetic analyses suggest acoustic wave velocity could be used as a selection criterion in breeding programs or as a screening tool to identify selection candidates to be sampled using more-precise but more expensive assessment tools such as NIR (Downes et al. 2009) and marker assisted selection (Thavamanikumar et al. 2011).
Selection based on AWV is likely to result in positive indirect responses in other wood properties, including MOE and basic density, as suggested by genetic correlation estimates from E. nitens (Blackburn et al. 2012; Blackburn et al. 2010; Blackburn et al. 2014). Similarly, in addition to pulp yield, QTL for AWV were co-located with numerous growth and other wood property traits within and among families, consistent with strong correlations among chemical wood properties in E. globulus (e.g. Stackpole et al. 2011). Although common breeding objective traits for pulpwood include pulp yield, wood density and diameter growth (Potts et al. 2013), chemical traits, such as the content of cellulose, lignin and extractives and the ratio of syringyl to guaiacyl subunits of lignin (S:G ratio), are also of economic importance, as they are either related to pulp yield or affect the cost and efficiency of pul** (Stackpole et al. 2011). Hence, co-location of QTL with density and chemical traits other than pulp yield coupled with the positive inter-correlations of AWV with pulp yield, cellulose and density bode well for the prospects of using AWV to select trees, not only for solid wood but also for pulpwood breeding objectives.
The phenotypic correlation between AWV and pulp yield (rp = 0.71, R2 = 0.50) in the quantitative genetic study implies that segregation of E. globulus logs into pulpwood classes based on acoustic properties is possible during or post harvest (Amishev et al. 2010). This correlation was stronger than previous estimates for E. nitens (Blackburn et al. 2012; rp = 0.39, R2 = 0.15; Downes et al. 2008; R2 = 0.25) and E. dunnii (Raymond et al. 2010; R2 = 0.25), possibly due to different assessment methods used. However, in all studies a significant and positive correlation between these traits was observed, conceivably driven by a positive correlation between AWV and fibre length and increased cellulose content and decreased lignin content corresponding with increased fibre length (refer to Raymond et al. 2010). Acoustic wave velocity has also been shown to correlate with cell wall microfibril angle in Eucalyptus dunnii (Dickson et al. 2003) and Pinus radiata (Sharma et al. 2015). Along with density, microfibril angle is a key trait affecting modulus of elasticity and thus stiffness, and has been shown to be affected by polymorphisms in the CCR gene (Thumma et al. 2005). The absence of co-location of AWV QTL with the CCR gene in the present case is noteworthy given the findings of Thumma et al. (2005).
The additive genetic correlation between AWV and diameter (0.58) was within the range of estimates for E. nitens reported by Blackburn et al. (2014; 0.18, 0.20, 0.51 and 0.71), Furthermore, the positive additive genetic correlations of diameter with cellulose content (0.48) and pulp yield (0.38) were favourable and in kee** with recent studies by Stackpole et al. (2010; 0.52 between diameter and pulp yield), Costa e Silva et al. (2009); 0.12 between diameter and pulp yield across multiple trials) and Apiolaza et al. (2005; 0.61 between diameter and cellulose content). However, these positive correlations were not significantly different from zero, except in the case of diameter with cellulose content in the current study. Furthermore, these results contrast with negative, albeit also non-significant, estimates published prior to 2006 (refer to Stackpole et al. 2010). Clarification of the direction and strength of correlations between diameter and these key pulpwood traits is required.
The strong genetic correlation between pulp yield and cellulose content (0.97) supports the findings of Apiolaza et al. (2005; 0.82) and Stackpole et al. (2010; 0.91). This indicates that tree breeders can make selections based on either NIR-predicted pulp yield or cellulose content with little impact on genetic gain.
The estimated narrow-sense heritability for AWV (0.26) was lower than most estimates from E. nitens open-pollinated base-population trials (0.16, 0.39, 0.44 and 0.74; Blackburn et al. 2014). Furthermore, NIR-predicted pulp yield and cellulose content heritabilities and CVa were lower than past estimates in E. globulus from open-pollinated trials (Apiolaza et al. 2005; Cotterill and Brolin 1997; Dean et al. 1990; Raymond and Schimleck 2002; Raymond et al. 2001a; Stackpole et al. 2010). It is not known if this low level of additive variation was an artefact of the assessment method (NIR on drill swarf samples extracted from near the cambium), which has not been extensively used. Lower heritability estimates for wood properties (e.g. pilodyn penetration, an indirect measure of basic density; Costa e Silva et al. 2009) in control-pollinated trials compared with open pollinated trials has previously been noted. However, in contrast to growth (Costa e Silva et al. 2010), there is no direct evidence from past studies of E. globulus wood properties for inbreeding depression affecting open-pollinated estimates of heritability (Volker et al. 2008) or of significant dominance variation. In the only other study to report a dominance variance for pulp yield, Costa e Silva et al. (2009) found that it hit the boundary of the parameter space at zero and that the epistatic variance was not significantly different from zero. Small and, again, non-significant dominance variances have been observed in the more widely studied wood property basic density (measured directly on wood cores or indirectly as pilodyn penetration; Costa e Silva et al. 2009; Li et al. 2007; Volker 2002). Furthermore, strong correlations observed between breeding values estimated from open-pollinated and control-pollinated trials (Volker 2002) and the absence of inbreeding depression (Hardner et al. 1998) also suggest that dominance genetic effects are of low importance relative to additive effects in this trait. In contrast, the near unity ratio of dominance to additive variance for diameter, reflected the findings of some past studies, most notably Li et al. (2007; refer to Hamilton et al. 2015a).
The ranking of genetic groups for pulp yield was not consistent with the findings of Stackpole et al. (2010), the largest study of the genetics of this trait in E. globulus, in which pulp yield of all mainland Australian subraces was lower than the pulp yield of the Bass Strait Islands and Tasmanian subraces, with the exception of the three subraces from northeastern Tasmania. In the present study, the Strezlecki genetic group from the mainland had the lowest pulp yield and cellulose content, and King Island (a Bass Strait island) ranked second highest. However, the Otways genetic group, from the mainland, had the highest pulp yield, and Tasmania ranked the second lowest. The low pulp yield of the Tasmanian genetic group was possibly explained by the inclusion of a number of northeastern Tasmanian families (Table 1). Furthermore, studies other than Stackpole et al. (2010) found the western Otways subrace (the subrace with the greatest contribution to our Otways genetic group; Table 1) to rank highly for pulp yield (Apiolaza et al. 2005; Raymond et al. 2001a). Cape Patton and Eastern Otways were also represented in the Otways genetic group, but there are no published pulp yield estimates for these subraces beyond the Stackpole et al. (2010) study. The stability of the ranking of subraces across sites for this key pulpwood trait requires further investigation.
It is possible that NIR predicted pulp yield and cellulose content in the current study were upwardly biased, as samples were extracted from the outer wood and both pulp yield and cellulose content increase from pith to cambium (Downes et al. 2012). Average NIR-predicted kraft pulp yield (54.7% for Strzelecki to 57.3% for Otways) was greater than those reported in other genetic studies (e.g. 51.6% from Apiolaza et al. 2005; 52.2%, pooled estimate, from Costa e Silva et al. 2009; 54.1% from Dean et al. 1990; 51.9, 52.2 and 51.9% from Raymond et al. 2001b; and 53.1% from Stackpole et al. 2010). The same was true for cellulose content (42.2% from Apiolaza et al. 2005; 46% from Cotterill and Brolin 1997; and 42.9, 42.1, and 40.4% from Raymond and Schimleck 2002).
5 Conclusion
We found that AWV is significantly genetically positively correlated with predicted pulp yield in E. globulus, consistent with the previous studies in E. nitens. Standing-tree AWV is used as an indirect measure of modulus of elasticity, a key trait for structural veneer and sawn timber. Selection on the basis of AWV is thus expected to provide genetic gains in wood properties favourable to both pulpwood and solidwood breeding objectives. However, prior to widespread adoption of AWV as a selection criterion in breeding programs, genetic correlations require verification across the species, breeding populations, environments, ages and methods of measurement.
References
Amishev D, Dowding B, Murphy G (2010) Challenges from incorporating acoustic technology on mechanical harvesters/processors for real-time wood stiffness assessment. FORMEC 2010 Forest Engineering: Meeting the Needs of the Society and the Environment (ISBN: 978–88–6129-569-8), Padova, Italy
Apiolaza LA, Raymond CA, Yeo BJ (2005) Genetic variation of physical and chemical wood properties of Eucalyptus globulus. Silvae Genet 54:160–166
Blackburn D, Hamilton M, Harwood C, Innes T, Potts B, Williams D (2010) Stiffness and checking of Eucalyptus nitens sawn boards: genetic variation and potential for genetic improvement. Tree Genet Genomes 6:757–765. doi:10.1007/s11295-010-0289-7
Blackburn D, Farrell R, Hamilton M, Volker P, Harwood C, Williams D, Potts B (2012) Genetic improvement for pulpwood and peeled veneer in Eucalyptus nitens. Can J For Res 42:1724–1732. doi:10.1139/X2012-105
Blackburn D, Hamilton M, Williams D, Harwood C, Potts B (2014) Acoustic wave velocity as a selection trait in Eucalyptus nitens. Forests 5:744–762. doi:10.3390/f5040744
Breitling R, Li Y, Tesson BM, Fu JY, Wu CL, Wiltshire T, Gerrits A, Bystrykh LV, de Haan G, Su AI, Jansen RC (2008) Genetical genomics: spotlight on QTL hotspots. Plos Genetics 4:ARTN e1000232. doi:10.1371/journal.pgen.1000232
Churchill G, Doerge R (1994) Empirical threshold values for quantitative trait map**. Genetics 138:963–971
Costa e Silva J, Borralho N, Araújo J, Vaillancourt R, Potts B (2009) Genetic parameters for growth, wood density and pulp yield in Eucalyptus globulus. Tree Genet Genomes 5:291–305. doi:10.1007/s11295-008-0174-9
Costa e Silva J, Hardner C, Tilyard P, Pires AM, Potts BM (2010) Effects of inbreeding on population mean performance and observational variances in Eucalyptus globulus. Ann For Sci 67:605. doi:10.1051/Forest/2010018
Cotterill PP, Brolin A (1997) Improving Eucalyptus wood, pulp and paper quality by genetic selection. Conferência IUFRO sobre Silvicultura e Melhoramento de Eucaliptos, pp. 1–13
Dean GH, French J, Tibbits WN (1990) Variation in pulp and papermaking characteristics in a field trial of Eucalyptus globulus. 44th Annual General Conference Appita, Rotorua, New Zealand, pp. B24.21-B24.33
Dickson RL, Raymond CA, Joe W, Wilkinson CA (2003) Segregation of Eucalyptus dunnii logs using acoustics. For Ecol Manag 179:243–251. doi:10.1016/S0378-1127(02)00519-4
Downes G, Ebdon N, Meder R, Joyce K, French J (2008) Standing tree measurement of acoustic velocity as a predictor of kraft pulp yield in E. nitens across two sites (ISBN: 978–1–920883-23-2). Forest and Wood Products Australia, Melbourne, Victoria, pp. 11
Downes GM, Meder R, Hicks C, Ebdon N (2009) Develo** and evaluating a multisite and multispecies NIR calibration for the prediction of Kraft pulp yield in eucalypts. Southern Forests 71:155–164. doi:10.2989/Sf.2009.71.2.11.826
Downes GM, Meder R, Bond H, Ebdon N, Hicks C, Harwood C (2011) Measurement of cellulose content, Kraft pulp yield and basic density in eucalypt woodmeal using multisite and multispecies near infra-red spectroscopic calibrations. Southern Forests 73:181–186. doi:10.2989/20702620.2011.639489
Downes GM, Harwood CE, Wiedemann J, Ebdon N, Bond H, Meder R (2012) Radial variation in Kraft pulp yield and cellulose content in Eucalyptus globulus wood across three contrasting sites predicted by near infrared spectroscopy. Can J For Res-Rev Can Rech For 42:1577–1586. doi:10.1139/X2012-083
Dutkowski GW, Potts BM (1999) Geographic patterns of genetic variation in Eucalyptus globulus ssp. globulus and a revised racial classification. Aust J Bot 47:237–263. doi:10.1071/BT97114
Freeman JS, Potts BM, Downes GM, Pilbeam D, Thavamanikumar S, Vaillancourt RE (2013) Stability of quantitative trait loci for growth and wood properties across multiple pedigrees and environments in Eucalyptus globulus. New Phytol 198:1121–1134. doi:10.1111/Nph.12237
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml User Guide Release 3.0 (ISBN: 1–904375–23-5), VSN International Ltd, Hemel Hempstead, UK
Hamilton MG, Potts BM (2008) Review of Eucalyptus nitens genetic parameters. N Z J For Sci 38:102–119
Hamilton M, Acuna M, Wiedemann J, Mitchell RP, David BM, Potts B (2015a) Genetic control of Eucalyptus globulus harvest traits. Can J For Res. doi:10.1139/cjfr-2014-0428
Hamilton MG, Blackburn DP, McGavin RL, Baillères H, Vega M, Potts BM (2015b) Factors affecting log traits and green rotary-peeled veneer recovery from temperate eucalypt plantations. Ann For Sci 72:357–365. doi:10.1007/s13595-014-0430-0
Hardner CM, Potts BM, Gore PL (1998) The relationship between cross success and spatial proximity of Eucalyptus globulus ssp. globulus parents. Evolution 52:614–618. doi:10.2307/2411096
Kirst M, Basten CJ, Myburg AA, Zeng ZB, Sederoff RR (2005) Genetic architecture of transcript-level variation in differentiating xylem of a eucalyptus hybrid. Genetics 169:2295-2303. doi: 10.1534/genetics.104.039198
Li Y, Dutkowski GW, Apiolaza LA, Pilbeam DJ, Costa e Silva J, Potts B (2007) The genetic architecture of a Eucalyptus globulus full-sib breeding population in Australia. For Genetics 12:167–179
Meder R, Downes GM, Brawner JT, Ebdon N (2010) Understanding radial variation to aid development of methods for in-field NIR assessment of Kraft pulp yield. TAPPI PEERS Conference, Norfolk, Virginia
Potts B, Hamilton M, Pilbeam D (2013) Genetic improvement of temperate eucalypts in Australia. In: Ipinza R, Borralho N (eds) El eucalipto en Chile, con énfasis en el Mejoramiento Genético Forestal. INFOR Instituto Forestal, Chile
Raymond CA, Schimleck LR (2002) Development of near infrared reflectance analysis calibrations for estimating genetic parameters for cellulose content in Eucalyptus globulus. Can J For Res 32:170–176. doi:10.1139/x01-174
Raymond CA, Schimleck LR, Muneri A, Michell AJ (2001a) Genetic parameters and genotype-by-environment interactions for pulp-yield predicted using near infrared reflectance analysis and pulp productivity in Eucalyptus globulus. For Genetics 8:213–224
Raymond CA, Schimleck LR, Muneri A, Michell AJ (2001b) Nondestructive sampling of Eucalyptus globulus and E. nitens for wood properties. III. Predicted pulp yield using near infrared reflectance analysis. Wood Sci Tech 35:203–215. doi:10.1007/s002260100092
Raymond CA, Thomas DS, Henson M (2010) Predicting pulp yield and pulp productivity of Eucalyptus dunnii using acoustic techniques. Aust For 73:91–97. doi:10.1080/00049158.2010.10676314
Sharma M, Apiolaza LA, Chauhan S, Mclean JP, Wikaira J (2015) Ranking very young Pinus radiata families for acoustic stiffness and validation by microfibril angle. Ann For Sci. doi:10.1007/s13595-015-0529-y
Stackpole DJ, Vaillancourt RE, Downes GM, Harwood CE, Potts BM (2010) Genetic control of kraft pulp yield in Eucalyptus globulus. Can J For Res 40:917–927. doi:10.1139/X10-035
Stackpole D, Vaillancourt R, Alves A, Rodrigues J, Potts B (2011) Genetic variation in the chemical components of Eucalyptus globulus wood. G3 1:151–159. doi: 10.1534/g3.111.000372
Thavamanikumar S, McManus LJ, Tibbits JFG, Bossinger G (2011) The significance of single nucleotide polymorphisms (SNPs) in Eucalyptus globulus breeding programs. Aust For 74:23–29. doi:10.1080/00049158.2011.10676342
Thumma BR, Nolan MR, Evans R, Moran GF (2005) Polymorphisms in cinnamoyl CoA reductase (CCR) are associated with variation in microfibril angle in Eucalyptus spp. Genetics 171:1257–1265. doi:10.1534/genetics.105.042028
Van Ooijen J, Kyazma B (2009) MapQTL 6. Software for the map** of quantitative trait loci in experimental populations of diploid species. Wageningen, Netherlands
Volker PW (2002) Genetics of Eucalyptus globulus, E. nitens and F1 hybrid. PhD, University of Tasmania
Volker PW, Potts BM, Borralho NMG (2008) Genetic parameters of intra- and inter-specific hybrids of Eucalyptus globulus and E. nitens. Tree Genet Genomes 4:445–460. doi:10.1007/s11295-007-0122-0
Acknowledgements
We thank the Western Australian Plantation Resources (WAPRES) for their substantial long-term commitment to the provision of site, growing of seedlings, trial establishment, stand maintenance, and tree measurement; and the Southern Tree Breeding Association (STBA) for the trial establishment and the provision of trial data. Emlyn Williams and Gavin Moran (CSIRO) designed the F1 field trials. We also thank John Wiedemann (WAPRES) for his technical support. This project utilised the University of Tasmania’s Central Science Laboratory (CSL) equipment and the assistance of CSL staff member Adam Smolenski.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Cooperative Research Centre for Forestry, National Centre for Future Forest Industries and the Australian Research Council (grants LP0884001 and LP140100506).
Additional information
Handling Editor: Jean-Michel Leban
Contribution of the co-authors Matthew G. Hamilton, Jules S. Freeman, David P. Blackburn, Geoffrey M. Downes, David J. Pilbeam and Brad M Potts undertook writing/editing the manuscript and all, except Matthew Hamilton and Brad Potts were involved in the data collection. Matthew Hamilton undertook the bulk of the quantitative genetic analyses. Jules S. Freeman undertook the bulk of the QTL analyses. Matthew Hamilton and Jules Freeman wrote the first draft of the manuscript. Brad Potts assisted in the genetic analyses and manuscript preparation. David J. Pilbeam was actively involved in the management of the trials over their life.
Rights and permissions
About this article
Cite this article
Hamilton, M.G., Freeman, J.S., Blackburn, D.P. et al. Independent lines of evidence of a genetic relationship between acoustic wave velocity and kraft pulp yield in Eucalyptus globulus . Annals of Forest Science 74, 17 (2017). https://doi.org/10.1007/s13595-017-0617-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-017-0617-2