Abstract
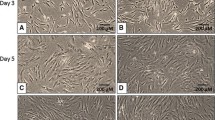
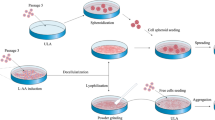
Human adipose-derived stem cells (hADSCs) are potential adult stem cells source for cell therapy. But hADSCs with multi-passage or cryopreservation often revealed poor growth performance. The aim of our work was to improve the activity of poor post-thaw hADSCs by simple and effective means. We describe here a simple method based on commercially available silicone micro-wells for creating hADSCs spheroids to improve viability and neural differentiation potential on poor post-thaw hADSCs. The isolated hADSCs positively expresse d CD29, CD44, CD105, and negatively expressed CD34, CD45, HLA-DR by flow cytometry. Meanwhile, they had adipogenic and osteogenic differentiation capacity. The post-thaw and post-spheroid hADSCs from poor growth status hADSCs showed a marked increase in cell proliferation by CKK-8 analysis, cell cycle analysis and Ki67/P27 quantitative polymerase chain reaction (qPCR) analysis. They also displayed an increase viability of anti-apoptosis by annexin v and propidium iodide assays and mitochondrial membrane potential assays. After 3 days of neural induction, the neural differentiation potential of post-thaw and post-spheroid hADSCs could be enhanced by qPCR analysis and western blotting analysis. These results suggested that the spheroid formation could improve the viability and neural differentiation potential of bad growth status hADSCs, which is conducive to ADSCs research and cell therapy.







Similar content being viewed by others
References
Abudusaimi A, Aihemaitijiang Y, Wang YH, et al. Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. J Int Med Res. 2011;39:1852–60.
Dong Y, Zhang Q, Li Y, et al. Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci. 2012;13:13605–20.
Liu S, Shao Y, Lin Q, et al. 7,8-Dihydroxy coumarin promotes chondrogenic differentiation of adipose-derived mesenchymal stem cells. J Int Med Res. 2013;41:82–96.
Tsai CC, Huang TF, Ma HL, et al. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transpl. 2013;22:413–22.
Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem cells. 2009;27:2624–35.
Konno M, Hamabe A, Hasegawa S, et al. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ. 2013;55:309–18.
Wang S, Qu X, Zhao RC. Mesenchymal stem cells hold promise for regenerative medicine. Front Med. 2011;5:372–8.
Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202.
Zhu X, Shi W, Tai W, et al. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived mesenchymal stem cells. Cell Tissue Res. 2012;350:277–87.
McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem cells. 2006;24:1246–53.
Yoon IS, Chung W, Sung JH, et al. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J Biosci Bioeng. 2011;112:402–8.
Santo VE, Duarte AR, Popa EG, et al. Enhancement of osteogenic differentiation of human adipose derived stem cells by the controlled release of platelet lysates from hybrid scaffolds produced by supercritical fluid foaming. J Control Release. 2012;162:19–27.
Bayati V, Sadeghi Y, Shokrgozar MA, et al. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell. 2011;43:359–66.
Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Harris LJ, Zhang P, Abdollahi H, et al. Availability of adipose-derived stem cells in patients undergoing vascular surgical procedures. J Surg Res. 2010;163:e105–12.
Baer PC, Doring C, Hansmann ML, et al. New insights into epithelial differentiation of human adipose-derived stem cells. J Tissue Eng Regen Med. 2013;7:271–8.
De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9.
Dicker A, Le Blanc K, Astrom G, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–90.
Locke M, Feisst V, Dunbar PR. Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem cells. 2011;29:404–11.
Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86.
Renzi S, Lombardo T, Dotti S, et al. Mesenchymal stromal cell cryopreservation. Biopreserv Biobank. 2012;10:276–81.
Freimark D, Sehl C, Weber C, et al. Systematic parameter optimization of a Me(2)SO- and serum-free cryopreservation protocol for human mesenchymal stem cells. Cryobiology. 2011;63:67–75.
Freimark D, Pino-Grace P, Pohl S, et al. Use of encapsulated stem cells to overcome the bottleneck of cell availability for cell therapy approaches. Transfus Med Hemother. 2010;37:66–73.
Wagh V, Meganathan K, Jagtap S, et al. Effects of cryopreservation on the transcriptome of human embryonic stem cell after thawing and culturing. Stem Cell Rev. 2011;7:506–17.
Stéphenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: is it time to change the strategy? World J Gastroenterol. 2010;16:1–14.
Page H, Flood P, Reynaud EG. Three-dimensional tissue cultures: current trend and beyond. Cell Tissue Res. 2013;352:123–31.
Garcion E, Halilagic A, Faissner A, et al. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–32.
Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–18.
Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93.
Song X, Zhu CH, Doan C, et al. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–7.
Huang YC, Chan CC, Lin WT, et al. Scalable production of controllable dermal papilla spheroids on PVA surfaces and the effects of spheroid size on hair follicle regeneration. Biomaterials. 2013;34:442–51.
Jun Y, Kang AR, Lee JS, et al. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials. 2014;35:4815–26.
Rajanahalli P, Meyer K, Zhu L, et al. Conversion of mouse fibroblasts to sphere cells induced by AlbuMAXI-containing medium. Front Biosci (Elite Ed). 2012;4:1813–22.
Guo Y, Liu Q, Yang Y, et al. The effects of ROCK inhibitor Y-27632 on injectable spheroids of bovine corneal endothelial cells. Cell Reprogram. 2015;17:77–87.
Li H, Dai Y, Shu J et al. Spheroid cultures promote the stemness of corneal stromal cells. Tissue Cell. 2015;47:39–48.
Martinello T, Bronzini I, Maccatrozzo L, et al. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci. 2011;91:18–24.
Vishnubalaji R, Al-Nbaheen M, Kadalmani B, et al. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012;347:419–27.
Dosier CR, Erdman CP, Park JH, et al. Resveratrol effect on osteogenic differentiation of rat and human adipose derived stem cells in a 3-D culture environment. J Mech Behav Biomed. 2012;11:112–22.
Chen TF, Wong YS. Selenocystine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generation. Int J Biochem Cell Biol. 2009;41:666–76.
Perelman A, Wachtel C, Cohen M, et al. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e403.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–8.
Liu Y, Jiang XH, Yu MK, et al. Switching from bone marrow-derived neurons to epithelial cells through dedifferentiation and translineage redifferentiation. Cell Biol Int. 2010;34:1075–83.
Yang XF, He X, He J, et al. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59.
Behr B, Tang C, Germann G, et al. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem cells. 2011;29:286–96.
Unek G, Ozmen A, Mendilcioglu I, et al. The expression of cell cycle related proteins PCNA, Ki67, p27 and p57 in normal and preeclamptic human placentas. Tissue Cell. 2014;46:198–205.
Nam BM, Kim BY, Jo YH, et al. Effect of cryopreservation and cell passage number on cell preparations destined for autologous chondrocyte transplantation. Transplant Proc. 2014;46:1145–9.
Ratcliffe E, Thomas RJ, Williams DJ. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br Med Bull. 2011;100:137–55.
Colwell AS, Borud LJ. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2008;121:701–2.
Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55.
Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–85.
Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–27.
Minonzio G, Corazza M, Mariotta L, et al. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology. 2014;69:211–6.
Rajagopal K, Chilbule SK, Madhuri V. Viability, proliferation and phenotype maintenance in cryopreserved human iliacapophyseal chondrocytes. Cell Tissue Bank. 2013;15:153–63.
Naaldijk Y, Staude M, Fedorova V, et al. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol. 2012;12:49.
Chinnadurai R, Garcia MA, Sakurai Y, et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports. 2014;3:60–72.
Francois M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensin. Cytotherapy. 2012;14:147–52.
Ragoonanan V, Hubel A, Aksan A. Response of the cell membrane-cytoskeleton complex to osmotic and freeze/thaw stresses. Cryobiology. 2010;61:335–44.
Galipeau J. The mesenchymal stromal cell dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8.
Pollock K, Sumstad D, Kadidlo D, et al. Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy. 2015;17:38–45.
Yu G, Floyd ZE, Wu X, et al. Isolation of human adipose-derived stem cells from lipoaspirates. Methods Mol Biol. 2011;702:17–27.
Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–8.
Song X, Zhu CH, Doan C, et al. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–7.
Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–18.
Garcion E, Halilagic A, Faissner A, et al. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–32.
Bayoussef Z, Dixon JE, Stolnik S, et al. Aggregation promotes cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. J Tissue Eng Regen Med. 2012;6:e61873.
Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–84.
Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS ONE. 2011;6:e18293.
Birenboim R, Markus A, Goldstein RS. Simple generation of neurons from human embryonic stem cells using agarose multiwall dishes. J Neurosci Methods. 2013;214:9–14.
Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–98.
Su G, Zhao Y, Wei J, Han J, et al. The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials. 2013;34:3215–22.
Acknowledgments
The authors thank for the support from the National Natural Science Foundation of China (No. 81371689), collaborated Grant of HK-Macao-TW of Ministry of Science and Technology (2012DFH30060) and the Natural Science Foundation of Guangdong Province (S2013010013391).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, X., Li, S., Ji, Q. et al. Enhanced viability and neural differential potential in poor post-thaw hADSCs by agarose multi-well dishes and spheroid culture. Human Cell 28, 175–189 (2015). https://doi.org/10.1007/s13577-015-0116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-015-0116-4