Abstract
In amyotrophic lateral sclerosis (ALS), motor neuron degeneration occurs simultaneously with systemic metabolic dysfunction and neuro-inflammation. The fibroblast growth factor 21 (FGF21) plays an important role in the regulation of both phenomena and is a major hormone of energetic homeostasis. In this study, we aimed to determine the relevance of FGF21 pathway stimulation in a male mouse model of ALS (mutated SOD1-G93A mice) by using a pharmacological agonist of FGF21, R1Mab1. Mice (SOD1-WT and mutant SOD1-G93A) were treated with R1Mab1 or vehicle. Longitudinal data about clinical status (motor function, body weight) and biological parameters (including hormonal, immunological, and metabolomics profiles) were collected from the first symptoms to euthanasia at week 20. Multivariate models were performed to identify the main parameters associated with R1Mab1 treatment and to link them with clinical status, and metabolic pathways involving the discriminant metabolites were also determined. A beneficial clinical effect of R1Mab1 was revealed on slow rotarod (p = 0.032), despite a significant decrease in body weight of ALS mice (p < 0.001). We observed a decrease in serum TNF-α, MCP-1, and insulin levels (p = 0.0059, p = 0.003, and p = 0.01, respectively). At 16 weeks, metabolomics analyses revealed a clear discrimination (CV-ANOVA = 0.0086) according to the treatment and the most discriminant pathways, including sphingolipid metabolism, butanoate metabolism, pantothenate and CoA biosynthesis, and the metabolism of amino acids like tyrosine, arginine, proline, glycine, serine, alanine, aspartate, and glutamate. Mice treated with R1Mab1 had mildly higher performance on slow rotarod despite a decrease on body weight and could be linked with the anti-inflammatory effect of R1Mab1. These results indicate that FGF21 pathway is an interesting target in ALS, with a slight improvement in motor function combined with metabolic and anti-inflammatory effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by the selective death of motor neurons, leading to the patient’s death 2 to 5 years after diagnosis [1]. Metabolic disturbances play an important role in different tissues, especially muscle and brain, in this disease [2]. Despite the progress made in the understanding of the pathophysiological mechanisms involved in ALS, the interactions among these pathways are not completely known. As no study has enabled the successful determination of a treatment, except for riluzole [3, 4] or edaravone [5], the research in drug development remains a priority for ALS.
Among the main pathophysiological mechanisms involved in ALS, neuro-inflammation and metabolic alterations have been thoroughly described, and several studies show a link between both mechanisms. Indeed, the signaling cascades activated by inflammatory cytokines can modulate insulin signaling, particularly in adipose tissue, liver, skeletal muscle, and the central nervous system [6, 7]. The fibroblast growth factor 21 (FGF21) plays a key role in energy metabolism and inflammatory response. FGF21 may act as an autocrine, paracrine, or endocrine molecule in the insulin-sensitizing effects. In this context and motivated by the necessity of targeting multiple mechanisms at once, we hypothesized that modulation of the FGF21 pathway could represent an interesting strategy for ALS management. We used R1Mab1 (Genentech USA, San Francisco, CA), an IgG humanized agonistic antibody directed to the D3 domain of the FGF receptor 1 (FGFR1), to induce attenuation of the metabolic disturbance and the pro-inflammatory effects observed in ALS.
Accordingly, we aimed to clarify whether activation of FGFR1 may be beneficial to ALS patients, especially via inflammatory and metabolic protective mechanisms. Thus, the effects of the FGF21 pathway were determined by its stimulation in an ALS mouse model (SOD1-G93A transgenic mice mSOD1) using a FGFR1 antibody (R1Mab1). Metabolomics and hormonal and immunological findings as well as evolution of motor symptoms in mSOD1 mice treated with R1Mab1 were analyzed. Beyond the determination of the putative protective effect of R1Mab1, we thoroughly assessed the mechanism of action of a therapeutic agent targeting two mechanisms of utmost importance in the context of ALS.
Materials and Methods
Animals
Twenty-four SOD1-G93A (mSOD1) male mice, on a C57Bl/6J genetic background, and 24 male SOD1-wild-type C57Bl/6J (WT) controls were acquired from Charles Rivers Laboratory (Burlington, CA; strains #004435 and #000664, respectively). They were housed in groups of 3–5 per cage in a virus-free barrier facility under a 12-h light/dark cycle, with ad libitum access to food and water.
All animal experiments followed current European Union regulations (Directive 2010/63/EU), as recommended by the regional ethics committee for animal experimentation (CEEA Val de Loire decision #19).
R1Mab1 Treatment
R1Mab1, IgG humanized monoclonal antibody with agonistic activity on the fibroblast growth factor receptor 1 (FGFR1), was graciously provided by Genentech (San Francisco, CA) under a Material Transfer Agreement. A loading dose of 3 mg/kg of body weight was first injected intraperitoneally (i.p.) into 12 mSOD1 and 14 WT mice 12 weeks after birth, followed by 1 injection of 0.5 mg/kg at 16 weeks after birth. 12 mSOD1 and 10 WT control mice received phosphate buffer saline (PBS) following the same schedule of treatment. The administration and sample collection protocol are shown on Fig. 1.
Flowchart for sample collection design, 3 mg/kg of body weight were first injected intraperitoneally into 12 mSOD1 and 14 WT mice 12 weeks after birth, followed by 1 injection of 0.5 mg/kg at 16 weeks after birth. 12 mSOD1 and 10 WT control mice received phosphate buffer saline following the same schedule. Submandibular collection of blood was performed at 8, 12, 16, and 20 weeks. Mice were euthanized 20 weeks
Sample Collection
Submandibular collection of blood (150-275 μL or 11 mL/kg body mass) was performed 8, 12, 16, and 20 weeks after birth, without the use of anesthesia. Mice were euthanized 20 weeks after birth by cervical dislocation.
Evaluation of Symptom Progression
Disease progression was assessed by monitoring body weight and locomotor performance. From the age of 7 weeks to the time of euthanasia, each animal in all experimental groups was weighed twice per week. The rotarod test was used to assess mouse locomotor performance once per week from 8 weeks of age to 13 weeks and twice per week from 13 weeks of age until week 20. Testing began by placing the mice on a rod (diameter = 3.5 cm) rotating at a constant speed of 25 rpm (fast rotarod). The time taken before the mice fell off the rod was recorded and used as a measure of motor function (test limited to 5 min maximum). Three trials were performed, and the longest time spent on the rod was recorded. From the 18th week on, another attempt was made at a speed of 15 rpm for 3 min (slow rotarod). The age of the mice at the first failure in this last attempt was also recorded and used as another locomotor performance outcome.
Hormone and Cytokine Assessment
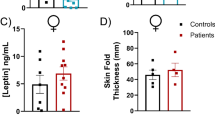
Serum samples (10 μL) collected from mice at 8, 12, 16, and 20 weeks of age were collected to measure IL-6, insulin, leptin, MCP-1, resistin, and TNF-α using a Milliplex MAP magnetic bead assay (Millipore, Billerica, MA) and Milliplex Analyzer (Luminex, Austin, TX). The detection limit was 3.2 pg/mL for each analyte after application of the manufacturer’s protocol. Data were analyzed using Milliplex Analyst 5.1 software.
Metabolomics Analysis
LC-HRMS analysis was performed as previously described [8, 9] after an extraction with 100 μL of methanol and using a UPLC Ultimate WPS-3000 system (Dionex, Germany) coupled to a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) and operated in positive (ESI+) and negative (ESI−) electrospray ionization modes (analysis for each ionization mode). Liquid chromatography was performed using a Phenomenex Kinetex 1.7 μm XB – C18 column (100 mm × 2.10 mm) maintained at 40 °C. Two mobile phase gradients (preceded by an equilibrium time of 3 min) were used. The gradient operated at a flow rate of 0.4 mL/min over a runtime of 26.5 min. Analyses were also performed on a hydrophilic interaction liquid chromatography (HILIC) column (100 mm × 2.10 mm, 100 Å). During the full-scan acquisition, which ranged from 58 to 870 m/z, the instrument operated at 70,000 resolution (m/z = 200). To validate the preanalytical and analytical steps of the experiment, quality control (QC) samples (mix of 15 samples analyzed) were analyzed as follows: coefficients of variation [CV% = (the standard deviation/mean) × 100] were calculated from all metabolites data. Metabolites having a CV in QCs > 30% were excluded from the final dataset. QCs were analyzed at the beginning of the run, every 10 samples and at the end of the run.
Accordingly, the final dataset contained only metabolites presenting low preanalytical and analytical variabilities.
For this targeted analysis, a library of standard compounds (Mass Spectroscopy Metabolite Library (MSML®) of standards, IROA Technologies™) was analyzed. The identity of each metabolite was characterized according to several criteria: 1) the retention time of the detected metabolite must be within ± 20 s of the standard reference, (2) the exact measured of molecular mass of the metabolite must be within a range of 10 ppm around the known molecular mass of the reference compound, and (3) the isotopic ratios of the metabolite must correspond to the standard reference. The signal value was calculated using Xcalibur® software (Thermo Fisher Scientific, San Jose, CA) by integrating the chromatographic peak area corresponding to the selected metabolite.
Gene Expression Analysis
Total RNA was extracted using Trizol from muscle samples (R1Mab1 mSOD1 n = 5; vehicle-treated mSOD1 n = 5) obtained at week 20 and then purified with DirectZol kit (Takara, reference R2052). One microgram of RNA was used to generate cDNA using a PrimeScript RT reagent kit (Takara, reference RR037A). β-Actin gene was used as reference gene. PCR amplifications were done in duplicates using a Sybrgreen Takion kit (Eurogentec reference UE-NSMT-B0701) and a LightCycler480 (Roche). Primers used for quantitative real-time PCR of genes including uncoupling protein 1 (UCP-1), peroxisome proliferator activated receptor α (PPARα), and PPARγ were based on that previously described [10] and are listed in Table S1. Data were normalized to the reference gene and to the control according to the 2−∆∆Ct method with Excel (Microsoft, Redmond, WA). Reverse transcriptase control, complementary DNA control, and positive PCR control were within the accepted range.
Statistical Analysis
Clinical and Biological Data
Body weight data were analyzed as the relative variation in body weight at each time and using the weight at the beginning of treatment (week 12) as the reference.
For each continuous variable (clinical, cytokine, and hormone findings), comparisons between subgroups were performed by Wilcoxon signed-rank test when the Student’s t test was not applicable.
The effect of time on continuous variable was analyzed using Friedman test when ANOVA was not applicable and was also evaluated between subgroups using 2-way ANOVA.
The significance threshold (p value) was set at 0.05 and was corrected for multiple tests when necessary with the Benjamini–Hochberg procedure.
Metabolomics Data
Metabolomics data were analyzed using multivariate analysis by Principal Component Analysis (PCA) and Orthogonal Partial Last Square Discriminant Analysis (OPLS-DA), with Simca P+ version 13.0 (Umetrics, Umea, Sweden) to 1) evaluate metabolic modification in mSOD1 mice treated with R1Mab1 versus placebo; 2) identify the metabolic modifications associated with R1Mab1 and specific to ALS conditions (after comparison with WT mice); and 3) determine the metabolites linked to the clinical outcome of treated mSOD1 mice.
PCA and OPLS-DA were performed for each time point (weeks 12, 16, and 20). The significance of the OPLS-DA model was evaluated by the CV-ANOVA test (based on F test to determine whether the variance of cross-validated residuals is significantly smaller than the variation of each Yi near the mean value of Y [11], with a significant threshold set at 0.05. The Q2Ycum and the R2Ycum values correspond to the goodness of prediction and the goodness of fit, respectively. We considered a model with Q2Y cum > 0.4 to be a sufficiently predictive model. The models were cross-validated by withholding one-seventh of the samples in seven simulations. The most relevant variables were determined from the variable importance for the projection (VIP) and loading values scaled as correlation coefficients (pcorr). VIP values represent the importance of the variable for the OPLS-DA models, and loadings characterize the relation between Y and X variables; they were considered relevant if VIP > 1 and pcorr > 0.4.
Venn diagrams were generated to highlight common and distinct metabolites identified from the multivariate models (including comparisons between mice groups).
Pathway Analysis
Pathway impact represents a combination of the centrality and pathway enrichment results; higher impact values represent the relative importance of the pathway, relative to all pathways included in the analysis. Metabolic pathways are represented as a network of chemical compounds with metabolites as nodes and reactions as edges. Major criteria are used to perform an informative analysis regarding the quality of pathway data. These data were downloaded from the KEGG database [12]. Chemical compounds and pathway topology information were obtained from the KEGG graph package [13], and the current library contains 874 metabolic pathways from mice.
The KEGG pathway database (http://www.genome.jp/kegg/pathway.html) was used with the Metaboanalyst tool to explore the highlighted metabolic pathways [Full size image
Moreover, we observed a trend to an upregulation of PPARγ in mSOD1 treated with R1Mab1 (n = 5) versus vehicle-treated mSOD1 mice (n = 5) (p = 0.052) (Fig. 9).
Gene expression of PPARγ in the quadriceps muscle. mRNA was isolated from quadriceps muscle of mSOD1 mice treated with PBS (Vt mSOD1) or R1Mab1 (R1 mSOD1) and subjected to qPCR analysis. Data represent means ± sem (n = 5). Relative expression values were evaluated with the 2−ΔΔCt method using actin as housekee** gene
No difference was found for PPARα mRNA between both groups.