Abstract
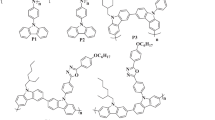
In the present work, we deal with some new organic materials having an extended conjugated electron system and providing tunable intramolecular charge transfer (ICT) properties. Firstly, the material structure, taken as a reference, is based on the N-ethylcarbazole motive as a central core having two bromo-distyrylbenzene units, denoted as M1, and has been synthesized and characterized principally using thermograviometric (ATG), absorption (OA), and emission spectroscopy (PL). Then, some derived compounds as symmetrical push–pull type materials with variations in their acceptor/anchor groups, containing four different kind of electron acceptor (A) groups and the ethylcarbazole as electron donor (D) part, denoted as CbzA1 → 4 were designed and elucidated. The impact of different electron-accepting strengths on the photophysical properties of carbazole derivatives is discussed. The correlation between structure properties of these materials has been well established. The withdrawing acceptor effect on their geometrical structure and optoelectronic properties was elucidated. In this framework, quantum calculations, based on density functional theory (DFT) and time-dependent DFT (TD-DFT) methods, in both gas and solution phases, agree well with the experimental results. The lowest lying absorption and fluorescence spectra are the signatures of intramolecular charge transfer (ICT) character. Additionally, N-donor substitution was used to modulate the photophysical properties of these push–pull compounds and thus the organic based photovoltaic device’s performance.
Graphical abstract

Photophysical properties and charcteristic parameters of D-(π-A)2 organic compounds

















Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
S.R. Forrest, M.E. Thompson, Organic electronics and optoelectronics. Chem. Rev. 107, 923–1386 (2007). https://doi.org/10.1021/cr0501590
R.D. Miller, E.A. Chandross, Materials for electronics. Chem. Rev. 110, 1–574 (2010). https://doi.org/10.1021/cr900384b
G.S. He, L.-S. Tan, Q. Zheng, P.N. Prasad, Chem. Rev. 108, 1245–1330 (2008). https://doi.org/10.1021/cr050054x
S. Ramkumar, S. Anandan, Synthesis of bianchored metal free organic dyes for dye sensitized solar cells. Dyes Pigm. 97, 397–404 (2013). https://doi.org/10.1016/j.dyepig.2013.01.014
M.S. Kang, D.H. Kim. 2020. On the Publication of the Special Issue on Flexible Optoelectronic Materials and Devices. Macromol. Res. 28 653
J.W. Ha, J.G. Jung, D.H. Ryu, S. Lee, C.E. Song, B. Lim, Y.J. Jung, J.M. Park, D.-H. Hwang, Thienoquinolinone-based acceptor-π-acceptor-type building block for polymer donors in organic solar cells. Macromol. Res. 31, 25–31 (2023). https://doi.org/10.1007/s13233-023-00112-1
J. Gong, K. Sumathya, Q. Qiao, Z. Zhou, Review on dye-sensitized solar cells (DSSCs): advanced techniques and research trends. Renew. Sustain. Energy Rev. 68, 234–246 (2017). https://doi.org/10.1016/j.rser.2016.09.097
C. Delesma, M. Robles, C. Amador-bedolla, J. Muñiz, The role of photoisomerization in the opto-electronic properties of organic photovoltaic materials: a DFT study. J. Photochem. Photobiol. 409, 113155 (2021)
P. Ledwon, P. Zassowski, T. Jarosz, M. Lapkowski, P. Wagner, V. Cherpak, P. Stakhira, Novel donor-acceptor carbazole and benzothiadiazole material for deep red and infrared emitting applications. J. Mater. Chem. C 4, 2219–2227 (2016). https://doi.org/10.1039/C5TC04183J
J. Kim, J. Park, D. Song, J. Jee, T. Gokulnath, S.C. Han, S.-H. **, J.W. Lee, BDT-based donor polymer for organic solar cells to achieve high efficiency over 15% for ternary organic solar cells. Macromol. Res. 31, 489–497 (2023). https://doi.org/10.1007/s13233-023-00117-w
N. Blouin, M. Leclerc, Poly(2,7-carbazole)s: Structure−property relationships. Acc. Chem. Res. 41, 1110–1119 (2008). https://doi.org/10.1021/ar800057k
J. Li, A.C. Grimsdale, Carbazole-based polymers for organic photovoltaic devices. Chem. Soc. Rev. 39, 2399–2410 (2010). https://doi.org/10.1039/B915995A
H.-P. Shi, J.-X. Dai, L.-W. Shi, L. Xu, Z.-B. Zhou, Y. Zhang, W. Zhou, C. Dong, Synthesis, photophysical and electrochemical properties of a carbazole dimer-based derivative with benzothiazole units. Spectrochimica Acta Part A 93, 19–25 (2012). https://doi.org/10.1016/j.saa.2012.02.087
W. Li, M. Otsuka, T. Kato, Y. Wang, T. Mori, T. Michinobu, 3,6-Carbazole vs 2,7-carbazole: a comparative study of hole-transporting polymeric materials for inorganic–organic hybrid perovskite solar cells. Beilstein J. Org. Chem. 12, 1401–1409 (2016). https://doi.org/10.3762/bjoc.12.134
Y.W. Lee, J. Yeop, J.Y. Kim, H.Y. Woo, Fullerene-based photoactive A-D-A triads for single-component organic solar cells: incorporation of non-fused planar conjugated core. Macromol. Res. 29, 871–881 (2021). https://doi.org/10.1007/s13233-021-9100-x
P. Ledwon, Recent advances of donor-acceptor type carbazole-based molecules for light emitting applications. Organ. Electron. 75, 105422 (2019). https://doi.org/10.1016/j.orgel.2019.105422
S. Ramkumar, S. Manoharan, S. Anandan, Synthesis of D-(π-A)2 organic chromophores for dye-sensitized solar cells. Dyes Pigm. 94, 503–511 (2012). https://doi.org/10.1016/j.dyepig.2012.02.016
H. Shi, Y. Cheng, W.-J. **g, J.-B. Cha, L. Fang, X.Q. Dong, C. Dong, Experimental and theoretical study of a new carbazole derivative having terminal benzimidazole rings. Spectrochimica Acta Part A 75, 525–532 (2010). https://doi.org/10.1016/j.saa.2009.11.003
R.J. Durand, S. Gauthier, S. Achelle, S. Kahlal, J.-Y. Saillard, A. Barsella, L. Wojcik, N. Le Poul, F.R. Le Guen, Incorporation of a platinum center in the pi-conjugated core of push-pull chromophores for nonlinear optics (NLO). Dalton Trans. 46, 3059–3069 (2017). https://doi.org/10.1039/C7DT00252A
R.J. Durand, S. Gauthier, S. Achelle, T. Groizard, S. Kahlal, J.Y. Saillard, A. Barsella, N. Le Poul, F.R. Le Guen, Push–pull D–π-Ru–π-A chromophores: synthesis and electrochemical photophysical and second order nonlinear optical properties. Dalton Trans 47, 3965–3975 (2018). https://doi.org/10.1039/C8DT00093J
R. Kacimi, M. Raftani, T. Abram, A. Azai, H. Ziyat, L. Bejjit, M.N. Bennani, M. Bouachrine, Theoretical design of D-π-A system new dyes candidate for DSSC application. Heliyon 7, e07171 (2021). https://doi.org/10.1016/j.heliyon.2021.e07171
H.P. Shi, Y. Cheng, W.-J. **g, J.-B. Chao, L. Fang, X.Q. Dong, C. Dong, Experimental and theoretical study of a new carbazole derivative having terminal benzimidazole rings. Spectrochimica Acta Part A 75, 525–532 (2010). https://doi.org/10.1016/j.saa.2009.11.003
A.D. Becke, Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993). https://doi.org/10.1063/1.464913
W. Kohn, L.J. Sham, Self-consistent equations including exchange and correlation effects. J. Phys. Rev. 140, A1133–A1138 (1965). https://doi.org/10.1103/physrev.140.a1133
P. Hohenberg, W. Kohn, Inhomogeneous electron Gas. J. Phys. Rev. 136, B864 (1964). https://doi.org/10.1103/PhysRev.136.B864
P.J. Stephens, F.J. Devlin, C.F. Chabalowski, M.J. Frisch, Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623 (1994). https://doi.org/10.1021/j100096a001
A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior. J. Phys. Rev. A 38, 3098–3100 (1988). https://doi.org/10.1103/PhysRevA.38.3098
A.D. Becke, A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993). https://doi.org/10.1063/1.464304
C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988). https://doi.org/10.1103/physrevb.37.785
M. Bursch, J.-M. Mewes, A. Hansen, S. Grimme, Best-practice dft protocols for basic molecular computational chemistry. Angew. Chem. 61, e202205735 (2022). https://doi.org/10.1002/ange.202205735
R. Ditchfield, W.J. Hehre, J.A. Pople, Self-consistent molecular-orbital methods IX An extended gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724 (1971). https://doi.org/10.1063/1.1674902
G. Saranya, P. Kolandaivel, K. Senthilkumar, Optical absorption and emission properties of fluoranthene, benzo[k]fluoranthene, and their derivatives. A DFT study. J. Phys. Chem. A 115, 14647–14656 (2011). https://doi.org/10.1021/jp208617s
M.J. Frisch, G.W. Trucks, H.B. Schlegel et al., Gaussian 09 revision A 02 (Gaussian Inc., Wallingford CT, 2016)
R.E. Stratmann, G.E. Scuseria, M.J. Frisch, An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 109, 8218 (1998). https://doi.org/10.1063/1.477483
R. Bauernschmitt, R. Ahlrichs, Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 256, 454 (1996). https://doi.org/10.1016/0009-2614(96)00440-X
F. Furche, R. Ahlrichs, Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 117, 7433–7447 (2002). https://doi.org/10.1063/1.1508368
T. Yanai, D.P. Tew, N.C. Handy, A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004). https://doi.org/10.1016/j.cplett.2004.06.011
M. Cossi, V. Barone, R. Cammi, J. Tomasi, Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 255, 327–335 (1996). https://doi.org/10.1016/0009-2614(96)00349-1
J.B. Foreman, M.H. Gordon, J.A. Pople, M.J. Frisch, Toward a systematic molecular orbital theory for excited states. J. Phys. Chem. 96, 135–149 (1992). https://doi.org/10.1021/j100180a030
T. Lu, F. Chen, Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). https://doi.org/10.1002/jcc.22885
K. Chaitanya, X.-H. Ju, B.M. Heron, Theoretical study on the light harvesting efficiency of zinc porphyrin sensitizers for DSSCs. RSC Adv. 4, 26621–26634 (2014). https://doi.org/10.1039/c4ra02473g
J. Wang, H. Li, N.-N. Ma, L.-K. Yan, Z.-M. Su, Theoretical studies on organoimido-substituted hexamolybdates dyes for dye-sensitized solar cells (DSSC). Dyes Pigm. 99, 440–446 (2013). https://doi.org/10.1016/j.dyepig.2013.05.027
U. Mehmood, S.-U. Rahman, K. Harrabi, I.A. Hussein, B.V.S. Reddy, Recent advances in dye sensitized solar cells. Adv. Mater. Sci. Eng. (2014). https://doi.org/10.1155/2014/974782
J.P. Perdew, R.G. Parr, M. Levy, J.L. Balduz, Density-functional theory for fractional particle number: derivative discontinuities of the energy. Phys. Rev. Lett. 49, 1691–1694 (1982). https://doi.org/10.1103/PhysRevLett.49.1691
J.L. Gázquez, A hardness and softness theory of bond energies and chemical reactivity. Theor. Comput. Chem. 5, 135–152 (1998). https://doi.org/10.1016/S1380-7323(98)80007-1
R.G. Parr, L.V. Szentpaly, S. Liu, Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999). https://doi.org/10.1021/ja983494x
M. Sun, Z. Cao, DFT and TD-DFT studies on osmacycle dyes with tunable photoelectronic properties for solar cells. Theor. Chem. Acc. 133, 1531 (2014). https://doi.org/10.1007/s00214-014-1531-4
J.L. Gázquez, A. Cedillo, A. Vela, Electrodonating and electroaccepting powers. J. Phys. Chem. A 111, 1966–1970 (2007). https://doi.org/10.1021/jp065459f
C.-N. Chuang, H.-J. Chuang, Y.-X. Wang, S.-H. Chen, J.-J. Huang, M.-K. Leung, K.-H. Hsieh, Polymers with alkyl main chain pendent biphenyl carbazole or triphenylamine unit as host for polymer light emitting diodes. Polymer 53, 4983–4992 (2012). https://doi.org/10.1016/j.polymer.2012.08.042
G.M. Upadhyay, H.R. Talele, S. Sahoo, A.V. Bedekar, Synthesis of carbazole derived aza[7]helicenes. Tetrahedron Lett. 55, 5394–5399 (2014). https://doi.org/10.1016/j.tetlet.2014.07.116
X. Liu, Y. Sun, Y. Zhang, N. Zhao, H. Zhao, G. Wang, X. Yu, H. Liu, A series of carbazole cationic compounds with large two-photon absorption cross sections for imaging mitochondria in living cells with two-photon fluorescence microscopy. J. Fluoresc. 21, 497–506 (2011). https://doi.org/10.1007/s10895-010-0736-8
D.F. O’Brien, P.E. Burrows, S.R. Forrest, B.E. Koene, D.E. Loy, M.E. Thompson, Hole transporting materials with high glass transition temperatures for use in organic light-emitting devices. Adv. Mater. 10, 1108–1112 (1998). https://doi.org/10.1002/(SICI)1521-4095
B.E. Koene, D.E. Loy, M.E. Thompson, Asymmetric triaryldiamines as thermally stable hole transporting layers for organic light-emitting devices. Chem. Mater. 10, 2235–2250 (1998). https://doi.org/10.1021/cm980186p
M.J. **ong, Z.H. Li, M.S. Wong, Synthesis and functional properties of star-burst dendrimers that contain carbazole as peripheral edges and triazine as central core. Aust. J. Chem. 60, 603–607 (2007). https://doi.org/10.1071/CH07038
P.J. Leenaers, A.J.L.A. Maufort, M.M. Wienk, R.A.J. Janssen, Impact of π-conjugated linkers on the effective exciton binding energy of diketopyrrolopyrrole-dithienopyrrole copolymers. J. Phys. Chem. C 124, 27403–27412 (2020). https://doi.org/10.1021/acs.jpcc.0c08768
T. Yanai, D.P. Tew, N.C. Handy, A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004). https://doi.org/10.1016/j.cplett.2004.06.011
T.D. Montiel, J.B. López, R.S. Rojo, D.G. Mitnik, Theoretical study of the effect of π-bridge on optical and electronic properties of carbazole-based sensitizers for DSSCs. Molecules 25, 3670 (2020). https://doi.org/10.3390/molecules25163670
P. Pounraj, P. Ramasamy, M.S., Pandian, The influence of π-linkers configuration on properties of 10-hexylphenoxazine donor-based sensitizer for dye-sensitized solar cell application–theoretical approach. J Mol Graph Model 102, 107779 (2021). https://doi.org/10.1016/j.jmgm.2020.107779
S. Roquet, A. Cravino, P. Leriche, O. Aleveque, P. Frere, J. Roncali, Triphenylaminethienylenevinylene hybrid systems with internal charge transfer as donor materials for heterojunction solar cells. J. Am. Chem. Soc. 128, 3459–3466 (2006). https://doi.org/10.1021/ja058178e
Z. Fahim, S. Bouzzine, A. Youssef, M. Bouachrine, M. Hamidi, Ground state geometries, UV/vis absorption spectra and charge transfer properties of triphenylamine-thiophenes based dyes for DSSCs: A TD-DFT benchmark study. Comput. Theor. Chem. 1125, 39–48 (2018). https://doi.org/10.1016/j.comptc.2018.01.002
H. Tian, X. Yang, R. Chen, R. Zhang, A. Hagfeldt, L. Sun, Effect of different dye baths and dye-structures on the performance of dye-sensitized solar cells based on triphenylamine dyes. J. Phys. Chem. C 112, 11023–11033 (2008). https://doi.org/10.1021/jp800953s
S. Mitroka, S. Zimmeck, D. Troya, J.M. Tanko, How solvent modulates hydroxyl radical reactivity in hydrogen atom abstractions. J. Am. Chem. Soc. 132, 2907–2913 (2010). https://doi.org/10.1021/ja903856t
J.-M. Ji, H. Zhou, H.K. Kim, Rational design criteria for D–π–A structured organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 6, 14518–14545 (2018). https://doi.org/10.1039/C8TA02281J
H.-H.G. Tsai, C.-J. Tan, W.-H. Tseng, Electron transfer of squaring-derived dyes adsorbed on TiO2 clusters in dye sensitized solar cells: a density functional theory investigation. J. Phys. Chem. C 119, 4431–4443 (2015). https://doi.org/10.1021/jp508034f
E. Steiner, Density-difference maps in quantum chemistry. Theoret. Chim. Acta 60, 561–572 (1982). https://doi.org/10.1007/BF00549611
R.G. Pearson, Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. 83, 8440–8441 (1986). https://doi.org/10.1073/pnas.83.22.8440
K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara, H. Arakawa, Molecular design of coumarin dyes for efficient dye-sensitized solar cells. J. Phys. Chem. B 107, 597–606 (2003). https://doi.org/10.1021/jp026963x
Z. Ning, Q. Zhang, W. Wu, H. Pei, B. Liu, H. Tian, Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J. Org. Chem. 73, 3791–3797 (2008). https://doi.org/10.1021/jo800159t
W. Sang-aroon, S. Saekow, V. Amornkitbamrung, Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 236, 35–40 (2012). https://doi.org/10.1016/j.jphotochem.2012.03.014
J.-P. Lellouche, R.R. Koner, S. Ghosh, N-Substituted carbazole heterocycles and derivatives as multipurpose chemical species: at the interface of chemical engineering, polymer and materials science. Rev. Chem. Eng. 29, 413–443 (2013). https://doi.org/10.1515/revce-2013-0023
K. Brunner, A.V. Dijken, H. Börner, J.J.A.M. Bastiaansen, N.N.M. Kiggen, B.M.W. Langeveld, Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes tuning the homo level without influencing the triplet energy in small molecules. J. Am. Chem. Soc. 126, 6035–6042 (2004). https://doi.org/10.1021/ja049883a
J.R. Reynolds, A.D. Child, J.P. Ruiz, S.Y. Hong, D.S. Marynick, Substituent effects on the electrical conductivity and electrochemical properties of conjugated furanylphenylene polymers. Macromolecules 26, 2095 (1993). https://doi.org/10.1021/ma00060a044
M. Helgesen, S.A. Gevorgyan, C.K. Frederik, Substituted 2,1,3-benzothiadiazole- and thiophene-based polymers for solar cells−introducing a new thermocleavable precursor. Chem. Mater. 21, 4669 (2009). https://doi.org/10.1021/cm901937d
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
AM: conceptualisation, methodology, analysis, visualization, writing original draft and editing. AH and ML: methodology, investigation, analysis, editing and writing the original draft. KA: supervision, administrative and auxiliary assistance and MBB: analysis of data, and revising of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hfaiedh, A., Labiedh, M., Mabrouk, A. et al. Synthesis, characterization and structure–property study of new push–pull carbazole materials. Macromol. Res. 31, 981–999 (2023). https://doi.org/10.1007/s13233-023-00182-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-023-00182-1