Abstract
Background
Cervical cancer (CC) is a danger to women’s health, especially in many develo** countries. Metabolomics can make the connection between genotypes and phenotypes. It provides a wide spectrum profile of biological processes under pathological or physiological conditions.
Method
In this study, we conducted plasma metabolomics of healthy volunteers and CC patients and integratively analyzed them with public CC tissue transcriptomics from Gene Expression Omnibus (GEO).
Result
Here, we screened out a panel of 5 metabolites to precisely distinguish CC patients from healthy volunteers. Furthermore, we utilized multi-omics approaches to explore patients with stage I-IIA1 and IIA2-IV4 CC and comprehensively analyzed the dysregulation of genes and metabolites in CC progression. We identified that plasma levels of trimethylamine N-oxide (TMAO) were associated with tumor size and regarded as a risk factor for CC. Moreover, we demonstrated that TMAO could promote HeLa cell proliferation in vitro. In this study, we delineated metabolic profiling in healthy volunteers and CC patients and revealed that TMAO was a potential biomarker to discriminate between I-IIA1 and IIA2-IV patients to indicate CC deterioration.
Conclusion
Our study identified a diagnostic model consisting of five metabolites in plasma that can effectively distinguish CC from healthy volunteers. Furthermore, we proposed that TMAO was associated with CC progression and might serve as a potential non-invasive biomarker to predict CC substage.
Impact
These findings provided evidence of the important role of metabolic molecules in the progression of cervical cancer disease, as well as their ability as potential biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cervical cancer (CC) is an important global public health issue, which ranks as one of the most common cancers and the top leading cause of cancer death in middle-aged women [1, 2]. CC has multiple risk factors including high-risk human papillomavirus (HPV) infection, unsanitary sexual behaviors, and long-term use of oral contraceptives [3]. Pap Smear and HPV tests are common screening procedures for cervical intraepithelial neoplasia (CIN) or CC [4, 5]. However, the gold standard for diagnosing and staging of CC depends on the tissue pathological characteristics of biopsy samples, which is obtained from an invasive pressure. According to the International Federation of Gynecology and Obstetrics (FIGO) guidelines, CC is divided into four stages, each of which contains, A and B sub-stages [6]. The precise and proper staging determination directly affects the treatment plans for CC patients [7].
Metabolomics can make the connection between genotypes and phenotypes [8]. It provides a wide spectrum profile of biological processes under pathological or physiological conditions. Small-molecule metabolites can not only act as biomarkers for disease conditions but also often participate in signal-transduction pathways to modulate phenotype [9]. The metabolomics-based screening is a robust and efficient way to discover candidate biomarkers for early cancer diagnosis, progression, and prognosis [10, 11]. Hasim et al. have performed plasma amino acid profiling in CC and CIN patients compared with healthy volunteers. It was found that CC and CIN patients had low levels of overall amino acid, and Lysine, tryptophan, cysteine and methionine metabolism pathways were also revealed differentially expressed in urine samples of CC patients in Liang et al.’s report [12, 13]. Yin et al. identified phosphatidylcholine and lysophosphatidylcholine in plasma acting as discriminate biomarkers to divide squamous cervical cancer (SCC) and uterine fibroid (UF) patients [14]. Yang et al. via integration of metabolomics and transcriptomics provided 5 candidate metabolites to diagnose CC [2.5 Cutoff value and AUC calculation The optimal cutoff value was calculated according to the ROC curve and Youden index analysis by SPSS. ROC curves were built to calculate AUC using pROC in R 3.6.3. R package survival and survminer were used to compute the Kaplan–Meier survival estimate and to plot survival curves. A log-rank test was used to compare two groups and determine statistical significance. Hela cell line was from FuHeng BioLogy (FH0314). Cells were cultured in RPMI1640 (11875093, Gibco) supplemented with 10% FBS and penicillin–streptomycin (15140163, Gibco) at 37 ℃ under 5% CO2. The cell suspension was incubated in a 96-well for 48 h and serum-starved for 12 h. Subsequently, cells were exposed to 0, 50, 100, 200, 400 and 800 µM TMAO (317594, Sigma-Aldrich) for 48 h. CCK-8 assay (CK04, DOJINDO) and EdU assay (KeyGEN BioTECH, KGA311) were performed to measure cell proliferation. Ten microliters of CCK-8 solution were added and incubated for 2 h and then absorbance was measured at 450 nm. Cells were added 10 μM EdU, fixed with 4% paraformaldehyde for 15 min, and supplemented with 0.5% Triton X-100 for 20 min. After washing cells with 3% BSA solution, the Click-It reaction buffer was incubated for 30 min in the shade. Subsequently, Edu stained cells were re-stained Hoechst 33342 for 30 min. After that, cells were imaged by a florescence microscope (IX71, Olympus). Cytotoxicity LDH assay was performed according to the manufacturer’s instructions (CK12, DOJINDO). These experiments were repeated 3 times.2.6 Survival analysis
2.7 Cell culture
2.8 Cell proliferation and cytotoxicity assay
3 Results
3.1 Plasma metabolic profiling revealed distinct metabolic alterations in CC patients
We performed LC–MS-based untargeted metabolomics of plasma samples collected from 43 CC patients and 27 healthy volunteers, with the detailed information shown in Table S1. The flowchart of this study is shown in Figure S1. PCA was performed on metabolomics data from healthy volunteers and CC patients, with component 1 (PC1) for 70.3% and component 2 (PC2) for 12.4% of the variation (Fig. 1A). The orthogonal partial least-squares discriminant analysis (OPLS-DA) model was built by the aforementioned cohort showing a clear separation between the two groups. The OPLS-DA score plot in the predictive (x-axis) and orthogonal (y-axis) components were 4.8 and 13.5% respectively (Fig. 1B). Differential metabolomics analysis revealed 51 metabolites (Fig. 1C, Table. S2), and the differentially expressed metabolites were enriched in carnitine metabolism, lipid metabolism, and amino acid metabolism (Fig. 1D). Amino acid dysfunction has been reported in several studies. Multiple amino acids and their derivates appeared to coordinate with each other (Fig. 1E). In addition, we integrated transcriptomics from GEO DataSets including tissue specimens of 24 normal volunteers and 28 CC patients. The network of differential metabolites and genes indicated the source of the metabolites may result from abnormal tumor metabolism (Fig. S3).
Metabolic molecular landscape in cervical cancer and control group. PCA (A) and OPLS-DA (B) score plot of metabolomics data. The ellipses display 95% confidence intervals. C Heatmap of differentially expressed metabolites using scaling peak intensity. D SMPDB (Small Molecule Pathway Database) enrichment analysis of altered metabolites. E Correlation plot of differentially expressed metabolites
3.2 A panel of five metabolites serves as potential biomarkers for the diagnosis of CC
We performed tenfold cross-validation LASSO model with training cohort including 70% of the cohort to get the optimal λ value (right dotted line, lambda = 0.136) which was 1 standard error (SE) of the minimum λ (left dotted line, lambda = 0.049) (Fig. 2A). Five metabolites (Cyclohexylamine, l-Carnitine, Val-Thr, Sinigrin, 5,6,7,8-tetrahydro-2-Naphthoic acid) contributing to the model most were finally selected and combined to form a predictive model (Fig. 2B). Structures of the five metabolites were confirmed by standard compounds (Fig. S2). The 5-metabolite panel behaved equally well for the training cohort, test cohort, and another independent validation cohort including 45 CC patients and 7 normal people (Fig. 2C–E). The peak intensities of metabolites in the panel changed significantly between the two groups (Fig. 2F–J). A low concentration of l-Carnitine in plasma increases fatigue and exhaustion in cancer patients [22]. However, sinigrin, a natural product mainly from cruciferous vegetables which are reported to have anti-cancer therapeutic activity [23], unexpectedly rises in the plasma of CC patients. As a reason, we hypothesize that the dynamics of gut microbiota may influence the metabolism of sinigrin [24]. Further investigation into the association between three other metabolites and cancer is warranted.
Identified metabolic biomarkers distinguishing cervical cancer from control. A Metabolites selection via LASSO regression analysis. ln(λ) is plotted on the x-axis while binomial deviance is plotted on the y-axis. B LASSO coefficient profiles of the 51 metabolites against ln(λ). C ROC curve for the training cohort. The AUC were 0.993 (95% CI 0.9808–1). D ROC curve for the test cohort. The AUC was 1 (95% CI 1–1). E ROC curve for the validation cohort. The AUC was 1 (95% CI 0.9279–1). F–J Boxplots of peak intensities of 5 potential biomarkers including cyclohexylamine, L-carnitine, Val-Thr, sinigrin, 5,6,7,8-Tetrahydro-2-Naphthoic acid with healthy volunteers and CC patients. The Student's t-test was used to evaluate the significance of the difference between the two groups
3.3 Multi-omics discrimination of I-IIA1 and IIA2-IV substages in CC
According to FIGO staging criteria, I-IIA1 and IIA2-IV are divided based on the greatest dimension of tumor size. For the smaller tumor size, radical hysterectomy is more suitable for patients in stage I-IIA1 and prevents additional chemoradiotherapy. To distinguish the molecular profiles of patients both in I-IIA1 and IIA2-IV, we carried out homemade metabolomics and TCGA-derived transcriptomics analysis. Gene sets enrichment analysis (GSEA) showed terms related to tumor progression (Fig. 3A–C). MYC is an oncogene that promotes cell proliferation as a transcription factor and Wnt/β-catenin signaling is activated in cancers [25, 26]. Using 11 differentially expressed metabolites, PCA analysis was performed with PC1 and PC2 adding up to 48.71%. The heatmap illustrated different patterns of top 11 significantly changed metabolites between two groups. We performed correlation analysis of differentially expressed metabolites of clinical indexes and found that tumor volume was associated with several metabolites such as TMAO and cancer biomarkers CA125 (Fig. 3F).
3.4 TMAO concentration reflects features of I-IIA1 and IIA2-IV patients as a biomarker
Concerning CC progression risk factors, we determined cutoff values based on the Youden index for TMAO and CA125 concentration as indicators. The cutoff value-based OR for classifying I-IIA1 and IIA2-IV indicated that TMAO was strongly correlated with CC progression while CA125 was weakly correlated (Fig. 4A). ROC curve analysis also served to confirm TMAO as a more accurate biomarker compared to CA125 on categorizing I-IIA1 and IIA2-IV patients (Fig. 4B, C and Fig. S4A, B). Survival analyses of I-IIA1 versus IIA2-IV show no significant differences in previous reports [27, 28]. However, in our cohort, stage IIA2-IV tended to represent a poor prognosis. Due to the small sample size, the statistical power may be not strong (Fig. S4C). We conducted targeted metabolomics on 27 plasma specimens. There was a significant increase in plasma concentration of TMAO in the IIA2-IV patients against the I-IIA1 patients (Fig. 4D, E and Fig. S5). Unexpectedly, the precursors of TMAO, carnitine, choline, and TMA, were not significantly changed between the two groups (Fig. S4D–F and Fig. 4E).
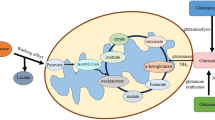
Identification of TMAO as a biomarker for different stages of cervical cancer patients. A Odds ratios with 95% CI and p-value, of TMAO and CA125 in predicting CC progression from I-IIA1 to IIA2-IV for all patients. B ROC curve of TMAO for the training cohort. The AUC was 0.872 (95% CI 0.7338–1). C ROC curve of TMAO for the test cohort. The AUC was 0.875 (95% CI 0.6578–1). D Boxplot of concentration of TMAO with I-IIA1 and IIA2-IV patients in derivation and validation cohorts. E Schematic diagram of sources of TMAO, showing increased TMAO and unchanged TMAO precursors in plasma of IIA2-IV patients compared to those of I-IIA1 patients
3.5 TMAO enhanced cancer cell proliferation in vitro.
To further investigate the clinical significance of increased TMAO concentration in plasma, we conducted functional studies at the cellular level in vitro. TMAO does not affect promoting endothelial cell viability under a series of concentrations from 50 to 1000 μM at different time points [20]. Considering that cancer cells may respond to different growth-stimulating signals from normal cells [29], we performed a CCK8 assay to analyze the proliferation of HeLa cells following treatment with a gradient concentration of TMAO in the culture medium for 48 h. The result showed that TMAO could modulate proliferation in a dose-dependent manner with a peak capacity at 400 μM (Fig. 5A). Under 400 μM treatment for 48 h, HeLa cells high exhibited cell counts and EdU positive cells (Fig. 5C) without increasing damaged cells through measuring lactate dehydrogenase activity (Fig. 5B). These results together indicated that TMAO has proliferation stimulating activity in HeLa cells.
TMAO functional analysis in vitro. A TMAO promoted HeLa cell proliferation in a dose-dependent manner. B HeLa cells treated with 400 μM TMAO were tested for LDH assay to assess TMAO cytotoxicity. C HeLa cells treated with 400 μM TMAO were tested for EdU assay. Representative immunofluorescence images of Hoechst, EdU, and the merged
4 Discussion
Sustaining proliferation and metabolic reprogramming are considered as hallmarks of cancer [30]. Metabolic disorders of tumor cells often induce abnormal metabolism of carbohydrates, lipids, amino acids, and other small molecules in cancer patients.
In this study, we mainly used metabolomics data to depict metabolic changes in onset and the progression of CC. We observed that the metabolism of a few core metabolites such as lipids and amino acids were altered in CC patients and the abnormal metabolic changes were accompanied by progression of CC. Due to its stable performance, LASSO is widely accepted and used to sort key variables and build clinical predictive models. Based on the differential metabolites we screened, we generated a diagnostic model consisting of five metabolites in plasma which was effective and non-invasive compared to the gold standard of histological diagnosis. The current study was limited to a single-center and has several other constraints. It should be acknowledged that the validation cohort exhibited an imbalance, which may not provide sufficient evidence for establishing a robust model.
As for the five metabolites, it remains unclear how these metabolites participate in the occurrence of CC and how metabolic changes in CC patients influence plasma metabolite abundance. However, there are some research reports on these 5 metabolic molecules in other diseases. Cyclohexylamine was identified as the serum metabolite biomarkers of the persons with type 2 diabetes with multiple complications [31]. l-carnitine was reported to decrease body weight and BMI through a variety of mechanisms, such as improving insulin resistance and may decrease appetite and food intake through a direct effect on hypothalamus [32,33,34,35]. Previous study showed that serum l-carnitine concentrations had a protective impact on overall, digestive system, and non-digestive system cancer risk [36]. Recent research supported decreases in Pro-CoA and its derivative propionyl-l-carnitine due to ALDH6A1 downregulation were tightly associated with hepatocellular carcinoma [37]. The 5,6,7,8-tetrahydro-2-Naphthoic Acid has been proven to be a degradation product of Naphthalene [38] and there were no reports of its association with diseases. Palaniraja et al. found that Valine tRNA levels and availability regulate complex I assembly in leukemia [39], Sai et al. proposed that L-valine may be a potential marker for the diagnosis of lung cancer [40]. However, there is currently a lack of reports on Val-Thr in disease research. The sinigrin harnessed like a prodrug catalyzed by myrosinase to the production of AITC, which induced cell apoptosis and arrested the growth of lung cancer cells [41].
Based on FIGO guidelines, 2A patients were separated into two groups to make different plans of treatment. For I-IIA1 patients, we perform radical surgery without chemoradiotherapy is supposed. For IIA2-IV patients, we considered combining chemoradiotherapy and surgery to take control of patients’ condition. To our knowledge, we comprehensively compared the metabolic profiles of the two groups through metabolomics and transcriptomics, finding that TMAO is a valuable diagnostic biomarker for discriminating the two groups.
TMAO is a small molecular compound. After consuming food that is rich in carnitine and choline, in the presence of gut bacteria, these substances are converted to TMA and further oxidized to TMAO in the liver resulting in an increased plasma concentration of TMAO. A high concentration of TMAO is associated with an increased risk of cardiovascular diseases through promoting vascular inflammation. Seldin et al. and Chen et al. reported that TMAO induces vascular inflammation through different pathways, by activating NLRP3 inflammasome and NF-κB signaling respectively [19, 20]. In addition, some studies provided shreds of evidence that there is a link between TMAO and cancer, especially CRC [21]. In recent years, the role of TMAO in cancer has gradually been discovered. Recent researches identified the microbiome-derived metabolite TMAO drived immune activation and boosted responses to immune checkpoint blockade in pancreatic cancer [42], TMAO or its precursor choline, may represent a novel therapeutic strategy to promote the efficacy of immunotherapy in triple-negative breast cancer [43]. The Gut Microbial Metabolite TMAO has also been shown to promotes inflammatory hepatocellular carcinoma by upregulating POSTN [44].
Our study first proposed that TMAO is related to CC progression and may serve as a potential non-invasive biomarker to predict CC substages. However, we did not detect any changes in TMAO precursors in plasma. We speculate that it may be due to abnormal FMO3 activity, which converts TMA to TMAO in the liver or reduced TMAO excretion. Although the pathological mechanisms remain unclear, we had displayed that TMAO had a proliferation promotion effect on HeLa cells to unravel the puzzle.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424.
What Are the Risk Factors for Cervical Cancer? [https://www.cdc.gov/cancer/cervical/basic_info/risk_factors.htm]
Cervical Cancer Screening Every 5 Years OK. Cancer discovery 2018, 8(10):1204.
Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, Guerra CE, Oeffinger KC, Shih Y-CT, Walter LC, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American cancer society. CA A Cancer J Clin. 2020. https://doi.org/10.3322/caac.21628.
Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, Kehoe ST, Konishi I, Olawaiye AB, Prat J, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129–35.
PA C, A J, A O, Lancet DLJ. Cervical cancer. Lancet. 2019;393(10167):169–82.
Fiehn O. Metabolomics–the link between genotypes and phenotypes. Plant Mol Biol. 2002;48(1–2):155–71.
Guijas C, Montenegro-Burke JR, Warth B, Spilker ME, Siuzdak G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat Biotechnol. 2018;36(4):316–20.
Benjamin D, Cravatt B, Nomura DJCm. Global profiling strategies for map** dysregulated metabolic pathways in cancer. Cell Metab. 2012;16(5):565–77.
Dinges S, Hohm A, Vandergrift L, Nowak J, Habbel P, Kaltashov I, Cheng LJNrU. Cancer metabolomic markers in urine: evidence, techniques and recommendations. Nat Rev Urol. 2019;16(6):339–62.
Hasim A, Aili A, Maimaiti A, Mamtimin B, Abudula A, Upur H. Plasma-free amino acid profiling of cervical cancer and cervical intraepithelial neoplasia patients and its application for early detection. Mol Biol Rep. 2013;40(10):5853–9.
Liang Q, Yu Q, Wu H, Zhu YZ, Zhang AH. Metabolite fingerprint analysis of cervical cancer using LC-QTOF/MS and multivariate data analysis. Anal Methods. 2014;6(12):3937–42.
Yin MZ, Tan S, Li X, Hou Y, Cao G, Li K, Kou J, Lou G. Identification of phosphatidylcholine and lysophosphatidylcholine as novel biomarkers for cervical cancers in a prospective cohort study. Tumour Biol. 2016;37(4):5485–92.
Yang K, **a B, Wang W, Cheng J, Yin M, **e H, Li J, Ma L, Yang C, Li A, et al. A comprehensive analysis of metabolomics and transcriptomics in cervical cancer. Sci Rep. 2017;7:43353.
Khan I, Nam M, Kwon M, Seo SS, Jung S, Han JS, Hwang GS, Kim MK. LC/MS-based polar metabolite profiling identified unique biomarker signatures for cervical cancer and cervical intraepithelial neoplasia using global and targeted metabolomics. Cancers. 2019. https://doi.org/10.3390/cancers11040511.
Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018. https://doi.org/10.3390/nu10101398.
Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60.
Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016. https://doi.org/10.1161/JAHA.115.002767.
Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017. https://doi.org/10.1161/JAHA.117.006347.
Chan CWH, Law BMH, Waye MMY, Chan JYW, So WKW, Chow KM. Trimethylamine-N-oxide as one hypothetical link for the relationship between intestinal microbiota and cancer—where we are and where shall we go? J Cancer. 2019;10(23):5874–82.
Matsui H, Einama T, Shichi S, Kanazawa R, Shibuya K, Suzuki T, Matsuzawa F, Hashimoto T, Homma S, Yamamoto J, et al. L-carnitine supplementation reduces the general fatigue of cancer patients during chemotherapy. Mol Clin Oncol. 2018;8(3):413–6.
Mazumder A, Dwivedi A, du Plessis J. Sinigrin and its therapeutic benefits. Molecules. 2016;21(4):416.
Krul C, Humblot C, Philippe C, Vermeulen M, van Nuenen M, Havenaar R, Rabot S. Metabolism of sinigrin (2-propenyl glucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model. Carcinogenesis. 2002;23(6):1009–16.
Schulze A, Oshi M, Endo I, Takabe K. MYC targets scores are associated with cancer aggressiveness and poor survival in ER-positive primary and metastatic breast cancer. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21218127.
Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60.
G G, S JP, T EP, C C, D G, A R, M RT. Stage IIA1 versus stage IIA2 cervical cancer: does the new staging criteria predict survival? Int J Gynecol Cancer. 2011;21(4):711–6.
Lai JC, Chou YJ, Huang N, Tsai JJ, Huang SM, Yang YC, Chang CL, Wang KL. Survival analysis of stage IIA1 and IIA2 cervical cancer patients. Taiwan J Obstet Gynecol. 2013;52(1):33–8.
Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML, Nawroth R, Sanchez-Garcia I, et al. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35:S25-54.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Yoshihiko Tomofuji KS, Kishikawa T, Shojima N, Hosoe J, Inagaki K, Matsubayashi S, Ishihara H, Watada H, Ishigaki Y, Inohara H, Murakami Y, Matsuda K, Okada Y, Yamauchi T, Kadowaki T. Identification of serum metabolome signatures associated with retinal and renal complications of type 2 diabetes. Commun Med. 2023. https://doi.org/10.1038/s43856-022-00231-3.
Xu Y, Jiang W, Chen G, Zhu W, Cui G. L-carnitine treatment of insulin resistance: a systematic review and meta-analysis. Adv Clin Exp Med. 2017;26(2):333–8.
Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9(6):756–61.
Nazary-Vannani A, Ghaedi E, Mousavi SM, Teymouri A, Rahmani J, Varkaneh HK. The effect of L-carnitine supplementation on serum leptin concentrations: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2018. https://doi.org/10.1007/s12020-018-1559-7.
A MA, C AHB, E MMD, F MESE, A AS, H EGG. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trials—ScienceDirect. Pharmacol Res. 2020. https://doi.org/10.1016/j.phrs.2019.104554.
Liu T, Liu C, Wang X, Wei Y, Li S, Song Y, Chen P, Liu L, Wang B, Shi H. The association of serum L-carnitine concentrations with the risk of cancer in Chinese adults with hypertension. Nutrients. 2022. https://doi.org/10.3390/nu14234999.
Sun J, Ding J, Shen Q, Wang X, Wang M, Huang Y, Zhang X, Zhu H, Zhang F, Wu D, et al. Decreased propionyl-CoA metabolism facilitates metabolic reprogramming and promotes hepatocellular carcinoma. J Hepatol. 2023;78(3):627–42.
Zhang X, Sullivan ER, Young LY. Evidence for aromatic ring reduction in the biodegradation pathwayof carboxylated naphthalene by a sulfate reducing consortium. Biodegradation. 2000;11(2–3):117–24.
Thandapani P, Kloetgen A, Witkowski MT, Glytsou C, Lee AK, Wang E, Wang J, LeBoeuf SE, Avrampou K, Papagiannakopoulos T, et al. Valine tRNA levels and availability regulate complex I assembly in leukaemia. Nature. 2022;601(7893):428–33.
Chen S, Gui R, Zhou X-H, Zhang J-H, Jiang H-Y, Liu H-T, Fu Y-F. Combined microbiome and metabolome analysis reveals a novel interplay between intestinal flora and serum metabolites in lung cancer. Front Cell Infect Microbiol. 2022;12:885093.
Tarar A, Alyami EM, Peng C-A. Eradication of myrosinase-tethered cancer cells by allyl isothiocyanate derived from enzymatic hydrolysis of sinigrin. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics14010144.
Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, Tang H-Y, Liu Q, Auslander N, Dang CV, et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol. 2022;7(75):0704.
Wang H, Rong X, Zhao G, Zhou Y, **ao Y, Ma D, ** X, Wu Y, Yan Y, Yang H, et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022. https://doi.org/10.1016/j.cmet.2022.02.010.
Wu Y, Rong X, Pan M, Wang T, Yang H, Chen X, **ao Z, Zhao C. Integrated analysis reveals the gut microbial metabolite TMAO promotes inflammatory hepatocellular carcinoma by upregulating POSTN. Front Cell Dev Biol. 2022;10:840171.
Funding
This work was supported by the Scientific Research Fund of Sichuan health and Health Committee (No. 21PJ085), Innovative Scientific Research Project of Medical Youth in Sichuan Province (Q20073), the Dazhou-Sichuan University Intelligent Medical Laboratory in Dazhou (2021CDDZ-26, 21ZDYF0029), The Key Projects fund of Science & Technology Department of Sichuan Province (2022YFS0588, 2022JDRC0069).
Author information
Authors and Affiliations
Contributions
F.Z. conceived the idea and designed the study. X.L., L.Z. and X.H. performed most of the experiments, and analyzed and interpreted data. Q.P., S.Z., J.T., J.W., and D.G., performed part of the experiments and data collection. F.Z. and X.L. wrote the original draft and revised the manuscript. J.W., D.G. and F.Z. revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study followed the guidelines of the ethics committee and was approved by the Dazhou Central Hospital Ethics Committee (IRB00000046-17005). All patients signed the informed consent.
Consent for publication
All authors agree to publish this paper.
Competing interests
The authors declare no potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12672_2024_948_MOESM1_ESM.pdf
Supplementary file 1: Fig S1. Overview flowchart of the study. Fig S2. MS/MS spectra of cyclohexylamine (A), L-carnitine (B), Val-Thr (C), sinigrin (D), 5,6,7,8-tetrahydro-2-Naphthoic acid (E), (upper) matching to standard compounds (lower). Fig S3. Knitting network with both differentially expressed metabolites (blue nodes) and DEGs (red nodes). Fig S4. The performance of TMAO is to distinguish between I-IIA1 and IIA2-IV stages. (A) ROC curve of CA125 for the training cohort. The AUC was 0.821 (95% CI: 0.6231-1). (B) ROC curve of CA125 for the test cohort. The AUC was 0.750 (95% CI: 0.2132-1). (C) Survival plot and risk table of I-IIA1 (blue) and IIA2-IV (red) group. TMAO levels were determined by cutoff value. P value of the log-rank test was 0.015. (D - F) Boxplots of concentration of carnitine, choline, and TMA with I-IIA1 and IIA2-IV patients in derivation and validation cohorts. Fig S5. The expression level of TMAO in different pathological stages.
12672_2024_948_MOESM2_ESM.docx
Supplementary file 2: Table S1 Characteristics of cervical cancer patients. Table S2 The 51 Differential metabolites between cervical cancer and control group. Table S3 Performance of two model in training, test and validation sets.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Zhang, L., Huang, X. et al. High-throughput metabolomics identifies new biomarkers for cervical cancer. Discov Onc 15, 90 (2024). https://doi.org/10.1007/s12672-024-00948-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-00948-8