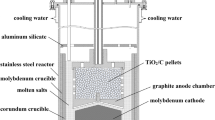
Abstract
Electrochemical reduction of metal oxides to metal has been gaining attention world over due to inherent advantages of simplicity of the process, energy savings and minimal environmental burden compared to conventional extraction technologies. More importantly, for reactive metals like titanium, R&D efforts have been increasingly put in for exploring the process for cost- and energy-effective production of metal. The electrochemical reduction of TiO2 essentially involves high-temperature (about 950 °C) cathodic treatment of the oxide granules/pre-forms in molten calcium chloride using graphite as anode. In the present work, the reduction of titanium dioxide to titanium metal has been extensively studied in different scales of operation. Among various process parameters, electrode potentials, DC voltage, bath temperature and current densities play a key role in the conversion of oxide to metal. Based on the experimental observations on variation of DC current, electrode potentials and CO/CO2 content in the vent gases, attempts have been made to develop improved understanding on mechanism of conversion and kinetic aspects of the reduction process. During scaling up of the reduction process, many process-related problems and technological issues such as incomplete conversion, characterization of the product and design of the cell with required electrode configuration, solid precipitates between electrodes and carbon fine deposition over the melt are encountered. This paper brings out various challenges that are being tackled in scaling up of the process along with important process observations that influence bulk kinetics of the process.







Similar content being viewed by others
References
Fray D J, Farthing T W and Chen G Z, Removal of oxygen from metal oxides and solid solutions by electrolysis in a fused salt, International Patent NoWO 9964638 (1999).
Chen G Z, Fray D J and Farthing T W, Nature 407 (2000) 361.
Chen G Z, and Fray D J, J Electrochem Soc 149 (2002) 455.
Benson L L, Mellor I, and Jackson M, J Mater Sci 51 (2016) 4250.
Jayashree M, Rath P C, Subbaiah T, Mishra P K, Paramguru R K, Transactions of the Indian Institute of Metals 62 (2009) 249.
Jayashree M, Krushna Gopal, Raja Kishore P and Barada K M, Metallurgical and materials Transactions B 43 (2012) 513.
Pinsheng Lai et al, Metallurgical & Materials Transactions B, TSM & ASM (2018).
Lebedev V A, Sal’nikov V I, Sizikov I A and Rymkevich D A, Russian Journal of Applied Chemistry 80 (2007) 1503.
Lebedev V A, Sal’nikov V I, Tarabaeu M V, Sizikov I A and Rymkevich D A, Russian Journal of Applied Chemistry 80 (2007) 1462.
Mohandas K S, Trans Inst Min Metall C 122 (2013) 1p95.
Kartik Rao et al, Development of new generation FFC pilot plant for production of low cost titanium and titanium alloys, in proceedings of 12th World conference on Titanium, June 19–24, 2011 China.
Govinda Rajulu G, Girish Kumar M, Hari Babu B, Srinivasa Rao K and Nagesh Ch R V S, Transactions of the Indian Institute of Metals 69 (2016) 999.
Govinda Rajulu G, Girish Kumar M, Hari Babu B, Srinivasa Rao K and Nagesh Ch R V S, Mater Trans 58 2017, The Japan Institute of Metals & Materials, 914.
Acknowledgements
The author is thankful to Dr. Vikas Kumar, Director, DMRL, for constant support extended to this developmental work and permitting to publish this technical paper. The author also wishes to acknowledge the hard work put in by the entire team of technical personnel of the group in the process development activity.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagesh, C.R.V.S. Challenges in the Development of Electrochemical Reduction of TiO2 to Titanium Metal. Trans Indian Inst Met 73, 2009–2014 (2020). https://doi.org/10.1007/s12666-020-01906-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-01906-y